Purulent infectious myositis
DOI:
https://doi.org/10.5935/Infect_evidencia/e20220816Keywords:
Pyomyositis, Staphylococcal infections, Anti-bacterial agents, Case ReportAbstract
Purulent infectious myositis is a rare infection of the skeletal muscle, with Staphylococcus aureus as the commonest primary etiologic agent, which occurs more frequently in men than women. Its mild and nonspecific initial manifestation can lead to a delayed diagnosis, resulting in complications such as endocarditis, osteomyelitis, and sepsis, subsequently affecting the patient’s prognosis. Herein, we present the case report of an adolescent female with no significant health history, who developed infectious myositis as a result of an insult secondary to mild trauma to the right upper limb. Medical diagnostic imaging was essential to both confirm the diagnosis and assess the extent of muscle involvement. Prolonged antibiotic therapy effectively treated the infection, and therefore no surgery was needed.
Downloads
INTRODUCTION
Purulent infectious myositis (PIM), formerly known as tropical pyomyositis, is an infection characterized by the presence of an intramuscular abscess due to bacterial infection, which is primarily attributed to Staphylococcus aureus1, 2. From an epidemiological standpoint, this disease typically affects men in their first or second decade of life2, 3. While the pathogenesis of PIM remains unclear, it is thought to be related to transient bacteremia that develops in the context of some preexisting muscle abnormality3.
Herein, we present a case report of PIM in an adolescent female with no significant health history, whose complementary medical diagnostic imaging evaluation, including magnetic resonance imaging (MRI) and positron emission tomography (PET), was essential to both confrm the diagnosis of PIM and to demonstrate the extent of muscle involvement. Despite the magnitude of involvement, prolonged antibiotic therapy was successful in treating the infection, therefore no surgery was needed.
CASE REPORT
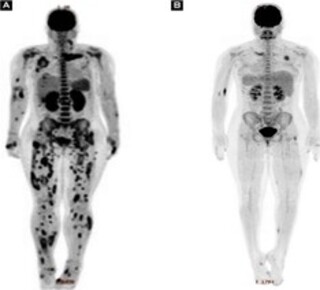
A 14-year-old female, with no known prior comorbidities, presented with pain in her left shoulder after she incurred a mild trauma during a recreational game seven days prior. The patient sought immediate medical assistance, but in the absence of any phlogistic signs upon physical examination performed during her initial evaluation, she was treated with an anti-infammatory medication to resolve the supposed muscle strain. After three days, the patient returned with fever of 39 °C, as well as worsening arthralgia. Blood counts and shoulder ultrasonography (US) were performed; however, the results showed no changes. Therefore, the patient was discharged again only with symptomatic medication. Her condition progressed beyond the initial arthralgia, however, and the patient began to have limited movement of other joints, such as her knees and hands, as well as pustular lesions erupted in her lower limbs. Twelve days after the onset of symptoms, she was taken to the emergency room at Emílio Ribas Institute of Infectious Diseases, presenting sleepy, feverish, eupneic, normotensive, with pustules on the lower limbs (Figure 1), swelling in the fourth metacarpophalangeal joint of the right hand (Figure 2), swelling and heat in the left knee, no phlogistic signs in the shoulders, and decreased limb strength (Grade 3). The remainder of the physical examination was normal. Considering the tentative diagnosis of reactive arthritis secondary to an infectious condition, antibiotic therapy with ceftriaxone was started. Table 1 shows the laboratory tests performed upon her admission. In the following days, the patient showed increased signs and symptoms (fever, arthralgia, limbs weakness), while new erythematous and painful subcutaneous nodules appeared in the trunk, upper limbs, and lower limbs. An US was performed on the knees bilaterally (which showed no relevant changes) as well as the abdomen (which showed hepatomegaly and nephropathy). The patient also underwent an MRI of the chest wall, which showed multiple small collections located in the belly of the left pectoralis major muscle, adjacent to the left sternoclavicular joint, as well as bone marrow signal alteration in the medial end of the left clavicle, suggesting osteomyelitis (images not available). The patient then underwent a PET scan with fluorodeoxyglucose (FDG) to better analyze the extent of the disease, the results of which showed multiple areas of densification in the muscular and subcutaneous planes with hypoattenuating centers in the thorax and pelvis, and dissemination to all four limbs (Figure 3a). Several blood culture samples collected during the patient’s hospitalization tested negative; however, in a single urine culture sample collected on the day of admission, there was growth of methicillin-susceptible Staphylococcus aureus (MSSA). The clinical, laboratory, and radiographic findings led to a diferential diagnosis of PIM. As a result of this diagnosis, the patient’s antimicrobial regimen was changed to oxacillin and clindamycin starting on the 11th day of her admission. In light of the disseminated abscesses, the pediatric surgery team chose not to perform multiple punctures and/or incisions for drainage, reserving this possibility in case of clinical worsening (sepsis), which did not happen. After eight weeks of intravenous antibiotic treatment, as well as progressive clinical, laboratory, and radiographic improvement (Figure 3b), the patient was discharged from hospital with a prescription of cephalexin concerning outpatient treatment of osteomyelitis.
Figure 1. Skin pustules on lower limb
Figure 2. Arthritis in the fourth metacarpophalangeal joint of the right hand
Figure 3. (a) PET scan with multiple densifications in muscle planes; (b) progress of PET scan after eight weeks of intravenous antibiotic therapy
| Blood count | Hemoglobin: 12.3 g/dL; Hematocrit: 34.8%; Total leukocytes: 24,300/mm3 (metamyelocytes, 2%; rods, 4%; neutrophils, 82%; lymphocytes, 5%; monocytes, 7%); platelets: 77,000/mm3 |
| C-reactive protein (CRP) | 396.60 mg/L (reference value 5–10 mg/dL) |
| Creatine phosphokinase (CPK) | 652 U/L |
| Transaminases (TGO/TGP) | 152 U/L; 202 U/L |
| Bilirubin | Total, 3.87 mg/dL; Direct, 3.21 mg/dL |
| Urea/Creatinine | 126 mg/dL; 2.21 mg/dL |
| Sodium/Potassium | 133 mmol/L; 4.2 mmol/L |
| Urine type 1 | pH, 6.0; blood, +++; protein, ++; leukocytes, 800,000/mL; erythrocytes, 250,000/mL; negative nitrites, with the presence of bacteria |
DISCUSSION
Tropical pyomyositis was initially described by Scriba4 in 1885; however, it wasn’t until 1971 that Levin et al.5 described it in the geographic context of temperate regions. Since its original description, due to the numerous cases documented globally, the regional nomenclature has been replaced by the term “PIM”,6 which refers to a subacute, deep infection of skeletal muscles, infectious disease that accounts for up to 4% of surgical hospital admissions in some countries 7, 8, 9. Early detection and treatment are considered to be the most efective interventions for PIM to minimize complications and reduce mortality, which can reach 23%8, 10. People with any type of immunodeficiency, diabetes mellitus, cancer, kidney failure, malnutrition, autoimmune disease, or a history of preexisting trauma are more predisposed to develop PIM. It is important, however, to highlight the potential of this disease to affect immunocompetent patients6, 7, 8, 10, as in the case described herein.
PIM can be categorized as primary or secondary, based on the route of infection. The former is intrinsically related to bacteremia, while the latter is associated with contiguous focus. The origin of the infection in primary PIM, however, occurs neither by continuity, nor by inoculation or penetrating injury6, 11, 12, as seen in the present case. The pathogenesis of PIM is thought to involve transient bacteremia associated with a prior skeletal muscle insult or traumatic injury, even a mild one such as that related to rigorous physical exercise. Other predisposing conditions have also been suggested, such as factors that affect skin integrity (atopic dermatitis, intravenous or intradermal drug use), nutritional deficiencies, and viral myositis. In most cases, however, the specific event which led to the development of PIM remains unclear1, 3, 6, 12.
When evaluating the disease topography and muscle groups involved, PIM can be categorized as focal or generalized6, 13. Any muscle group can be affected, and most abscesses are solitary, with only 12-40% of cases being multifocal14. The muscle groups most affected are the glutes, quadriceps, and iliopsoas14, 15, although some studies have also reported the involvement of the abdominal wall, cervical, foot, fank, forearm, and calf muscles2, 3, 14, 16. In the case of the patient presented herein, she had extensive and varied multifocal involvement, including the hand, which, according to relevant literature, is the rarest segment to be affected, corresponding to less than 1% of the cases reviewed3.
The primary form of PIM has three distinct progressive stages, which represent the progression from diffuse infammation to the formation of a localized abscess, which may or may not cause sepsis3, 6. The first stage, known as the invasive stage, occurs over a period of 10–21 days, with general nonspecific symptoms such as fever, vague muscle aches, and poorly localized edema, sometimes described as “woody induration” - few patients present with this stage. The second stage is the purulent or suppurative stage, and involves chills along with sustained fever, focal muscle pain, and increased edema. During this phase, exudate develops, which progresses to abscess formation. In this stage, a needle aspiration procedure can reveal purulent fluid. Most patients present at this stage. Finally, the third, or late stage, is characterized by obvious local signs, such as erythema and fuctuation, as well as systemic manifestations, including clinical signs of sepsis1, 2, 6. Complication percentages range from 9 to 66%, with sepsis being the most serious one. If not treated, contiguous or hematological dissemination may occur, which may subsequently progress to extra-muscular complications such as meningitis, myelitis, necrotizing fasciitis, septic pulmonary embolism, intra-abdominal abscesses, renal failure, pericarditis, cardiac tamponade, and osteomyelitis, among others6.
It can be difficult to diagnosis PIM in its early stages due to the lack of overlying skin changes combined with nonspecific findings on physical examination. Therefore, a high index of suspicion is necessary to achieve an early diagnosis, as diferential diagnoses include fever of undetermined etiology, orthopedic muscle conditions such as muscle strain, rupture, or hematoma, tumors such as osteosarcoma, osteomyelitis, septic arthritis, and thrombophlebitis. In cases where the abdominal muscles are affected, there may even be acute abdominal mimics1, 3, 6, 15. In the laboratory, the acute phase biomarkers are increased, in addition to leukocytosis with neutrophilic predominance in the complete blood count tests; muscle enzymes such as creatine phosphokinase (CPK) and aldolase can be normal or slightly elevated3, 9, 13, 15, 16. Infectious agent are not frequently identifed in blood cultures, occurring in up to 35% of cases1, 11, 12, 17. The culture of any purulent material obtained by the puncture or drainage of abscesses, on the other hand, may be negative in 15–30% of samples1. In cases where it is possible to identify the microorganism, Staphylococcus aureus is the most common6, 15, 18, found in up to 90% of the isolates1, 6, 17. Other gram-positive and negative agents, such as aerobes, anaerobes, mycobacteria, and fungi, have also been reported1, 3, 11, 14.
Medical diagnostic imaging plays a key role in the early and accurate diagnosis and evaluation of PIM. Radiographs have limited function due to their low sensitivity, and are therefore more useful in excluding other processes, such as osteomyelitis or orteosarcoma3, 18. US is very useful in the detection of superficial collections, in addition to allowing the guided puncture or drainage of abscesses1, 3, 6. Computed tomography (CT) and MRI are more sensitive in terms of demonstrating swelling of the affected muscle(s), corresponding to necrosis or collections. These lesions may reveal a contrast-enhanced peripheral ring, consistent with an abscess capsule which develops during the suppurative stage of the disease. MRI plays an essential role in timely and early diagnosis, particularly in the initial phase of the disease, allowing for the precise localization and improved delineation of the extent of muscle involvement18. While gallium or technetium scintigraphy can detect multifocal disease, single-photon emission computed tomography (SPECT) and PET scan can also be used for the same purpose, albeit at a higher cost6, 18.
Abscess drainage should be performed in conjunction with an appropriate antimicrobial therapy for the treatment of PIM. When there is an early diagnosis, that is, one in the first stage, the disease can be treated solely with antibiotic therapy, especially in children6, 7. The case described by Niamane et al.13 also showed therapeutic success with the exclusive use of antibiotic therapy as we have reported in the case presented herein, a decision which was determined by the multifocal distribution of PIM and the fact the patient was immunocompetent. The total recommended treatment time for PIM of bacterial etiology is 4-6 weeks, with the first 1-2 weeks of intravenous antibiotic therapy3, 6, 15 using an anti-staphylococcal drug resistant to inactivation by penicillinase1 - in selected cases, anti-methicillin-resistant Staphylococcus aureus (MRSA) coverage is required6.
CONCLUSION
The case presented herein clearly illustrates an incidence of PIM as a potentially serious and widespread disease affecting an immunocompetent adolescent female, having no direct relationship with any infectious point of entry, and with the most common etiologic agent (S. aureus) found only in urine culture. Despite the extensive distribution of affected muscle groups and the lack of surgical drainage of multiple abscesses, the patient showed good clinical improvement with prolonged antibiotic therapy.
“This case report deserved an official declaration of acknowledgement and ethical approval by its insttution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conficts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientfic community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Atribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Chauhan S, Jain S, Varma S, Chauhan SS. Tropical pyomyositis (myositis tropicans): current perspective. Postgrad Med J. 2004 Mai;80(943):267-70.
2. Chiedozi LC. Pyomyositis. Review of 205 cases in 112 patients. Am J Surg. 1979 Fev;137(2):255-9.
3. Bickels J, Ben-Sira L, Kessler A. Wientroub S. Primary pyomyositis. J Bone Joint Surg Am. 2002 Dez;84(12):2277-86.
4. Scriba J. Beitrang zur, Aetiologie der myositis acuta. Deutsche Zeit Chir. 1885;22:497-502 apud Chauhan S, Jain S, Varma S, Chauhan SS. Tropical pyomyositis (myositis tropicans): current perspective. Postgrad Med J. 2004;80:267-70.
5. Levin MJ, Gardner P, Waldvogel F. An unusual infection due to Staphylococcus aureus. N Engl J Med. 1971 Jan;284(4):196-8.
6. Habeych ME, Trinh T, Crum-Cianflone NF. Purulent infectious myositis (formerly tropical pyomyositis). J Neurol Sci. 2020 Jun;413:116767. DOI: https://doi.org/10.1016/j.jns.2020.116767
7. Gubbay AJ, Isaacs D. Pyomyositis in children. Pediatr Infect Dis J. 2000 Out;19(10):1009-12.
8. Ngor C, Hall L, Dean JA, Gilks CF. Factors associated with pyomyositis: a systematic review and metaanalysis. Trop Med Int Health. 2021 Out;26(10):1210-9.
9. Akman I, Ostrov B, Varma BK, Keenan G. Pyomyositis: report of three patients and review of the literature. Clin Pediatr. 1996 Ago;35(8):397-401.
10. Gibson RK, Rosenthal SJ, Lukert BP. Pyomyositis. Increasing recognition in temperate climates. Am J Med. 1984 Out;77(4):768-72.
11. Small LN, Ross JJ. Tropical and temperate pyomyositis. Infect Dis Clin North Am. 2005 Dez;19(4):981-9.
12. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008 Jul;21(3):473-94.
13. Niamane R, Jalal O, El Ghazi M, Hssaida R, Had A. Multifocal pyomyositis in an immunocompetent patient. Joint Bone Spine. 2004 Nov;71(6):595-7.
14. John BM, Patnaik SK. Multifocal pyomyositis. Med J Armed Forces India. 2007 Abr;63(2):191-2.
15. Comegna L, Guidone PI, Prezioso G, Franchini S, Petrosino MI, Filippo PD, et al. Pyomyositis is not only a tropical pathology: a case series. J Med Case Rep. 2016 Dez;10:372. DOI: https://doi.org/10.1186/s13256-016-1158-2
16. Taguchi BB, Francisco JA, Campos PTR, Teixeira CO, Teixeira MAB. Piomiosite tropical: correlação anatomoclínica. Relato de caso. Rev Bras Clin Med. 2013 Abr/Jun;11(2):194-6.
17. Brown JD, Wheeler B. Pyomyositis. Report of 18 cases in Hawaii. Arch Intern Med. 1984 Set;144(9):1749-51.
18. Theodorou SJ, Theodorou DJ, Resnick D. MR imaging findings of pyogenic bacterial myositis (pyomyositis) in patients with local muscle trauma: illustrative cases. Emerg Radiol. 2007 Jun;14(2):89-96.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















