Adherence to treatment as a prognostic factor for histoplasmosis in a patient with AIDS
DOI:
https://doi.org/10.5935/2764-734X.e20230617Keywords:
Histoplasmosis, Acquired immunodeficiency syndrome, Cooperation and adherence to treatment, Case ReportAbstract
The clinical management of histoplasmosis is dependent on the recognition of risk factors, their early diagnosis, and adequate adherence to treatment. Although histoplasmosis is a potentially serious disease, it is curable. The aim of this study was to report a case of histoplasmosis in a patient with AIDS, which progressed with unfavorable clinical repercussions due to poor adherence to treatment and lack of effective outpatient follow-up after several hospitalizations.
Downloads
INTRODUCTION
Histoplasmosis is a fungal disease caused by inhalation of Histoplasma capsulatum spores1. This disease is distributed worldwide; however, data related to its incidence in Brazil are imprecise. This is due to the fact that only hospitalizations need to be reported to the Ministry of Health and diagnosis of histoplasmosis is challenging2,3. Most infected patients present an asymptomatic or self-limited condition, and in 0.05% cases, the infection progresses to severe conditions4. Although rare, the disseminated form takes the course of a systemic granulomatous disease that can progress to death, depending on the intensity of exposure, host immunity, and delay in diagnosis1,3. However, the fungal infection itself may trigger the impaired immunological status of those infected, or aggravate it. Therefore, early diagnosis is crucial to ensure that patients receive adequate therapy to achieve better survival5,6.
The objective of this study was to report the case of a patient with acquired immunodeficiency syndrome (AIDS) and histoplasmosis that, although potentially curable, progressed unfavorably as a result of the lack of adherence to the proposed treatments and follow-ups, thereby allowing recurrences and facilitating the occurrence of concomitant secondary infections.
CASE REPORT
The patient was a 38-year-old caucasian man born in and originating from São Paulo, Brazil, who had an incomplete high school education. He was a mechanic, a smoker of 19 pack-years, and a former drinker and user of illicit drugs. The patient was diagnosed with AIDS 10 years ago and currently receives antiretroviral therapy (ART), albeit irregularly. The patient frequently visited fishing grounds on the edges of the Anhanguera Highway, in the State of São Paulo. The patient presented to the hospital with the main complaint of “skin sores.” According to the patient’s history, he was hospitalized for the first time April 2019 due to skin lesions. The lesions were diagnosed as histoplasmosis according to the results of a skin biopsy mentioned in a histopathological report, which described a “mild chronic suppurative inflammatory process with a granulomatous outline associated with fungal cells compatible with H. capsulatum”). The patient had no associated respiratory symptoms and reported having received treatment with an “antibiotic in the vein” (possibly amphotericin B); however, he had evaded hospitalization at that time.
The documents presented by the patient included a medical report from July 2019 (from another healthcare facility) that reported a short period of hospitalization. During this period, the patient was prescribed amphotericin B and RIPE (rifampicin, isoniazid, pyrazinamide, and ethambutol). The patient clarified that this was the third time he had been diagnosed with tuberculosis; however, he had never received treatment for the same. Details related to how this diagnosis of tuberculosis was established were absent, and the report stated that the ART regimen was changed to efavirenz instead of dolutegravir. This report also included a TCD4+ lymphocyte count (LTCD4+) of 1 cell/mm3 and a viral load (VL) of 532,535 copies/mL, and stated the following: “Due to the imminent risk of a new hospital evasion, we opted for early discharge with itraconazole 400 mg/day.”
In October 2019, the patient visited another hospital (the third) because of the worsening of his skin lesions since approximately 40 days prior; furthermore, he had reduced the daily dose of itraconazole to 200 mg/day on his own. The report of this third hospitalization included a LTCD4+ count of 2.6 cells/mm3 and a VL of 81,100 copies/mL, and treatment with amphotericin B was resumed for 14 days. Considering that the patient showed a “gradual and evident improvement of the lesions,” he was discharged with itraconazole 400 mg/day, in addition to ART and the regimen for tuberculosis already in the maintenance phase (with rifampicin and isoniazid).
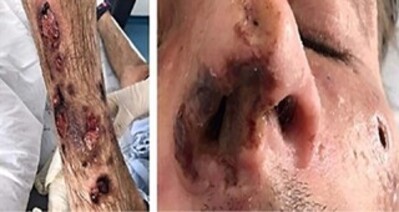
The patient sought another emergency room in January 2020 (the fourth different hospital) due to a new worsening of the skin lesions (documented in Figure 1), which presented as shallow, irregularly shaped ulcers covered by crusts, located on the nose wing, nasal septum, around the nostrils, and on both legs and arms. On this occasion the patient reported for the first time feeling intense pain in the skin lesions, as well as anorexia and a weight loss of approximately 10 kg in 15 days. The patient showed no fever nor respiratory symptoms. A new treatment with liposomal amphotericin B (50 mg/day) was restarted, replaced after two weeks by itraconazole (loading dose of 200 mg three times a day for three days, followed by 200 mg twice a day). New tests revealed an LTCD4+ count of 1.97 cells/mm3 and a VL of 36,500 copies/mL, as well as anemia with a hemoglobin concentration of 6.9 g/dL, which resulted in the transfusion of two units of red blood cell concentrate. The patient was discharged from the hospital after 30 days due to improvements in the lesions and the pain; ART, the double regimen for tuberculosis, and itraconazole 400 mg/day were maintained. The patient was scheduled to return the following month to undergo a myelogram. He collected this test in February 2020; the report showed hemodilution, with no evidence of spinal cord infiltration. However, the patient did not return to the subsequent scheduled outpatient appointments.
Figure 1. Ulcerated and crusted skin lesions on the lower limb, upper limb, and face (January 2020).
The patient returned only 4 months later (in June 2020) to the same last emergency room with the skin lesions again ulcerated and painful, in addition to presenting reduced bilateral visual acuity for two months and dysphagia for 7 days, managing to ingest only a pasty diet. After fundoscopy, ophthalmic cytomegalovirus was diagnosed (diffuse hemorrhagic lesions and areas of pallor in the retina), for which the patient was prescribed treatment with intravenous ganciclovir, 5 mg/kg every 12 hours. At the time of this new hospitalization, the patient had an LTCD4+ count of 4 cells/mm3 and a VL of 447 copies/ml. Upper digestive endoscopy revealed moderate enanthematous pangastritis and gradeB distal erosive esophagitis, suggesting esophageal candidiasis. Although the patient did not report respiratory complaints, a computed tomography (CT) of the chest (Figure 2) revealed bilateral, sparse, non-calcified nodular lesions, some of which surrounded by a slight amount of ground-glass infiltrate. The largest of these lesions, in the left lower lobe, had a diameter of 1.9 cm. No mediastinal lymphadenopathy was observed.
Figure 2. Computed tomography images of the chest (June 2020) showing bilateral, sparse, noncalcified nodular lesions (red arrows), interpreted as fungal infection.
These findings were considered compatible with fungal infection, and the patient was prescribed fluconazole. During hospitalization, the patient presented abdominal cramps and watery diarrhea, and then progressed with intermittent fever peaks, hypotension, bradycardia, tachypnea, tremor of the extremities, and mental confusion. The patient received antibiotic therapy with vancomycin and meropenem, and liposomal amphotericin B (50 mg/day) was introduced to replace fluconazole, which was on its 6th day of prescription. However, the clinical picture was aggravated through hemodynamic instability and the need for vasoactive drugs, in addition to a worsening of the respiratory pattern. Proportional palliative care was prioritized, and the patient died on the 15th day of this last hospitalization. The chronological sequence of treatments, recurrences, and outcome of the case are summarized in Table 1.
| 1st HOSPITALIZATION: APRIL 2019 | 2nd HOSPITALIZATION: JULY 2019 | 3rd HOSPITALIZATION: OCTOBER 2019 | 4th HOSPITALIZATION: JANUARY 2020 | 5th HOSPITALIZATION: JUNE 2020 | |
|---|---|---|---|---|---|
| LENGTH OF HOSPITAL STAY | <1 week | <1 week | 14 days | 30 days | 15 days |
| MAIN COMPLAINT | Skin lesions | Skin lesions | Skin lesions | Painful lesions and weight loss | Amaurosis and dysphagia |
| LTCD4+ | Unavailable | 1.0 cells/mm3 | 2.6 cells/mm3 | 1.9 cells/mm3 | 4.1 cells/mm3 |
| VIRAL LOAD FOR HIV | Unavailable | 532,535 copies/mL | 81,100 copies/mL | 36,500 copies/mL | 447 copies/mL |
| OTHER RELEVANT EXAMINATIONS | Skin biopsy: diagnosis of histoplasmosis | Diagnosis of pulmonary tuberculosis | — | Hemoglobin: |
Fundoscopia: CMV oftálmica TC de tórax: nódulos bilaterais Endoscopia: candidíase |
| TREATMENT DURING HOSPITALIZATION | Amphotericin B (incomplete) |
Amphotericin B (incomplete) Started RIPE |
Amphotericin B (14 days) |
Amphotericin B (14 days) + Itraconazole Blood transfusion |
Fluconazole followed by amphotericin B Broad-spectrum antibiotic therapy + ganciclovir |
| PRESCRIPTION FOR DISCHARGE | Evaded |
Itraconazole RIPE ART |
Itraconazole R+I ART |
Itraconazole R+I ART |
Death |
DISCUSSION
In Latin America, aside from Brazil, histoplasmosis is prevalent in Argentina, Colombia, Venezuela, Panama, and French Guiana. This disease is most often associated with HIV patients in French Guiana and Colombia, wherein 70% of patients experience co-infection7. In Brazil, histoplasmosis is considered endemic to the Northeast, Central-West, Southeast, and South regions, especially in rural areas with wild animals2,3. In the state of Rio de Janeiro, up to 90% of the population is exposed to it2.
The pulmonary aspiration of spores of the fungus H. capsulatum, if the spores resist the host’s defenses, provides a favorable environment for their growth and reproduction, including within phagocytic cells, which can promote their dissemination to lymph nodes and other organs8.
The main risk factor is exposure to contaminated soil, as in construction, mining, fishing grounds, e.g., the case of the patient reported herein, or ecotourism activities in caves9. The risk of progression to more severe forms increases in older adults and immunosuppressed individuals, and this is associated with the increase in cases of disseminated disease that have been observed since the 1980s2,9. The prevalence of disseminated histoplasmosis in immunosuppressed patients is at least 10 times higher than in immunocompetent persons, i.e., the infection is more severe and there is a high prevalence of concomitant diseases, especially in HIV-seropositive patients10.
In comparison to the clinical pictures described in the literature, the patient in this report presented only some of the symptoms most frequently associated with histoplasmosis, including weight loss, anorexia, fever, abdominal pain, and diarrhea4,11. Other common findings were not present, including hepatosplenomegaly, lymphadenopathy, myalgia, and arthralgia. Notably, changes in the laboratory test results, including pancytopenia and increased lactic dehydrogenase, were absent. Skin manifestations are nonspecific; however, they are notably more common in Latin American countries—including Brazil—when compared with the USA2,11,12. Regarding gastrointestinal manifestations, those presented by this patient may have occurred as a result of the dissemination of the fungus through the blood4; however, the hypothesis of other etiologies or co-infections cannot be eliminated, including tuberculosis or the cytomegalovirus diagnosed at the last hospitalization. The miliary pattern observed in histoplasmosis chest images is usually more frequent than the nodules shown in Figure 24,11,12, which may explain why the current patient did not present respiratory complaints until the final phase, i.e., when dyspnea was probably a consequence of secondary pulmonary infection.
Many cases of histoplasmosis are known to be diagnosed only after death, highlighting the lack of diagnostic suspicion in the presence of a highly nonspecific clinical picture2, due to which the definitive diagnosis of histoplasmosis is completely dependent on targeted histological and laboratory tests7.
In this patient, the disease was confirmed through a biopsy of a skin lesion performed in the first hospital visit. This is one of the gold-standard methods to diagnose histoplasmosis2. This biopsy was not thoroughly reviewed because all the four hospitals considered it to be reliable and consistent with the clinical picture. However, in the histopathological analysis, differentiating between H. capsulatum and other morphologically similar agents is important, especially Candida glabrata, Cryptococcus neoformans, Cryptococcus gattii, Leishmania spp., Pneumocystis jirovecii, Toxoplasma gondii, and Trypanosoma cruzi7.
Another possible diagnostic technique is the microscopic examination of biological specimens including fluids, lymph nodes, bone marrow, liver, or any focus of infection4,11, as well as their seeding in an appropriate culture medium (especially Sabouraud agar), including fragments of skin biopsies (which was not performed in this case). Culture is typically positive in 75%–85% of cases of disseminated disease4.
Some less invasive and faster diagnostic methods can also be used, including the detection of antibodies and plasma antigens2. Antigen testing may be particularly useful in HIV-positive patients because of low seroconversion rates2; however, it is not widely available12. In the disseminated form of histoplasmosis, its detection in the urine is more sensitive than the detection of antigens in the blood, which are present in 90% of the patients analyzed. Molecular biology techniques through polymerase chain reaction (PCR) show immense potential for the diagnosis of this disease12.
Considering the high mortality of histoplasmosis, treatment is indicated to all patients with the disease13 and involves an induction phase and a maintenance phase14. For hospitalized patients with severe complications, intense immunosuppression, clinical instability, sepsis, pancytopenia, or high fever, induction with amphotericin B is initiated because of its greater capacity to eradicate fungemia. In patients with mild to moderate symptoms, without central nervous system involvement, itraconazole is the drug of choice14,15. Fluconazole has a lower in vitro effect than itraconazole, in addition to a lower reduction in mortality and higher chances of relapse16. In the present report, fluconazole was the drug initially prescribed at the last hospitalization in June 2020; however, this drug should not be the first choice. In a prospective, multicenter, open-label, non-randomized study of 49 patients with HIV and mild or moderately severe histoplasmosis, more than a quarter of them had disease progression after fluconazole induction therapy, whereas approximately one third relapsed after maintenance with daily doses of this same medication17. Moreover, the development of antifungal resistance was observed during fluconazole therapy17.
The American guidelines recommend starting the treatment of severe conditions with liposomal amphotericin B, switching to itraconazole after 1–2 weeks13,18, in accordance with the dosage prescribed for this patient in October 2019. In mild to moderate cases, a multicenter study showed resolution of clinical symptoms in 85% of cases after 12 weeks of therapy with itraconazole alone14. Notably, a period of one year is recommended as the ideal treatment duration16. The importance of outpatient follow-up is highlighted, among other reasons, to ensure favorable adherence to medication. However, this was not observed in the patient in this report and certainly contributed to the unfavorable outcome.
Regarding antifungal prophylaxis for the prevention of primary infection by H. capsulatum, no evidence of its efficacy has been reported thus far13.
Co-infection with histoplasmosis and pulmonary tuberculosis is relatively frequent, although in this case the diagnosis of these diseases was not established concomitantly. Indeed, whether the diagnosis of tuberculosis was actually confirmed remains unknown. The two diseases have a similar clinical picture, and both agents remain latent in the body, allowing the reactivation of viable etiological foci after years11. A systematic review of cases published in Brazil reported co-infection with Mycobacterium tuberculosis in 10.37% of patients diagnosed with histoplasmosis2.
CONCLUSION
The management of histoplasmosis depends on its early diagnosis and adequate adherence to treatment, considering that it is a potentially serious but curable disease. The current report illustrates a case where, in addition to the evident epidemiology, the diagnosis was established immediately and appropriately, reinforced by each new medical intervention that indicated hospitalization for systemic treatment. However, the lack of outpatient follow-up, aggravated by uncontrolled poor adherence to treatment, led to the unfavorable outcome of this infection in an immunosuppressed patient, a lesson that highlights the importance of prioritizing post-discharge care.
References
1. Fortaleza SCB, Lopes SKA, Bandeira TJ, Nogueira TNAG, Holanda MA. Histoplasmose disseminada aguda em indivíduo imunocompetente. J Bras Pneumol. 2004;30(3):270-3.
2. Almeida MA, Almeida-Silva F, Guimarães AJ, Almeida-Paes R, Zancopé-Oliveira RM. The occurrence of histoplasmosis in Brazil: a systematic review. Int J Infect Dis. 2019;86:147-56.
3. Simms A, Kobayashi T, Endelman L, Sekar P. Disseminated histoplasmosis presenting as bilateral lower extremity paresis. Int J Infect Dis. 2020;95:265-7.
4. Marsilla MM, Khairunisa AA, Azyani Y, Petrick P. Disseminated histoplasmosis mimicking an acute appendicitis. Malays J Pathol. 2019;41(2):223-7.
5. Shahani L. Disseminated histoplasmosis mimicking relapsed chronic lymphocytic leukaemia. BMJ Case Rep. 2018; 2018:1-4.
6. Ferguson-Paul K, Park C, Childress S, Arnold S, Ault B, Bagga B. Disseminated histoplasmosis in pediatric kidney transplant recipients — a report of six cases and review of the literature. Pediatr Transplant. 2018;22(7):e13274. DOI: https://onlinelibrary.wiley.com/doi/full/10.1111/petr.13274
7. Azar MM, Loyd JL, Relich RF, Wheat LJ, Hage CA. Current concepts in the epidemiology, diagnosis, and management of histoplasmosis syndromes. Semin Respir Crit Care Med. 2020;41(1):13-30.
8. Mittal J, Ponce MG, Gendlina I, Nosanchuk JD. Histoplasma capsulatum: mechanisms for pathogenesis. Curr Top Microbiol Immunol. 2019;422:157-91.
9. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-15.
10. Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016;30(1):207-27.
11. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115-32.
12. Falci DR, Monteiro AA, Caurio CFB, Magalhães TCO, Xavier MO, Basso RP, et al. Histoplasmosis, an underdiagnosed disease affecting people living with HIV/AIDS in Brazil: results of a multicenter prospective cohort study using both classical mycology tests and histoplasma urine antigen detection. Open Forum Infectious Diseases [Internet]. 2019;6(4):ofz073. Available from: https://academic.oup.com/ofid/article/6/4/ofz073/5319214
13. Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-25.
14. Wheat J, Hafner R, Korzun AH, Limjoco MT, Spencer P, Larsen RA, et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trial Group. Am J Med. 1995;98(4):336-42.
15. Dismukes WE, Bradsher Junior RW, Cloud GC, Kauffman CA, Chapman SW, George RB, et al. Itraconazole therapy for blastomycosis and histoplasmosis. NIAID Mycoses Study Group. Am J Med. 1992;93(5):489-97.
16. Ferreira MS, Borges AS. Histoplasmose. Rev Soc Brasil Med Trop. 2009;42(2):192-8.
17. Wheat LJ, Connolly P, Smedema M, Brizendine E, Hafner R; AIDS Clinical Trials Group and the Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Emergence of resistance to fluconazole as a cause of failure during treatment of histoplasmosis in patients with acquired immunodeficiency disease syndrome. Clin Infect Dis. 2001;33(11):1910-3.
18. Wheat J. Histoplasmosis. Experience during outbreaks in Indianapolis and review of the literature. Medicine (Baltimore). 1997;76(5):339-54.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















