Nasal hypertrophic herpes in a patient with HIV/AIDS
DOI:
https://doi.org/10.5935/2764-734X.e20230928Keywords:
AIDS-Related Opportunistic Infections, Skin Diseases, Viral, Antiviral Agents, Imiquimod, herpes simplex, Plastic Surgery Procedures, Case ReportAbstract
Hypertrophic lesions caused by herpes simplex virus are uncommon presentations usually described in immunocompromised patients. These conditions are often challenging, both in their diagnostic and management. Therapeutic failures after use of first-line antivirals are common and other treatment strategies are frequently necessary, such as the use of immunomodulators and even surgical resection. Here we describe the case of an immunocompromised patient who was diagnosed with a hypertrophic herpes (HH) lesion on the nose, refractory to conventional treatments.
Downloads
INTRODUCTION
Skin and mucosal lesions are common presentations of herpes simplex virus (HSV) infection. In most cases, these lesions are described as painful erythematous shallow papules, vesicles, and ulcers1. The hypertrophic or nodular, tumor-like, verrucous, vegetative, or exophytic form stands out within the spectrum of atypical presentations of the disease. HSV lesions of this form are characteristically voluminous and can be confused with other clinical conditions, especially neoplasms, which reinforces the importance of prompt identification and establishment of the correct therapeutic strategy2.
Individuals with chronic immunosuppression, such as those infected with HIV, are most likely to present hypertrophic mucocutaneous lesions due to HSV. In addition, first-line antiviral medications are frequently not sufficient to control the disease, particularly in these patients3. Here, we report a case of hypertrophic herpes refractory to conventional therapeutic options in a patient living with HIV/AIDS.
CASE REPORT
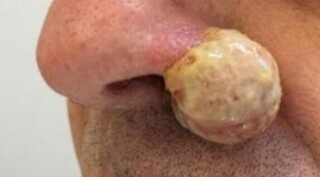
A 46-year-old unemployed and cisgender man presented to the emergency room complaining of a “wound” on the nose for 3 months, with insidious progression. He had acquired AIDS for over 20 years, with a history of good adherence to antiretroviral therapy (ART), currently in regular use of lamivudine, zidovudine, darunavir, ritonavir, and dolutegravir. The last HIV viral load measurements were consistently undetectable while the last CD4 lymphocyte count tests showed values > 200 cells/mm3. The patient had a history of multiple episodes of small blisters and painful ulcers occurring in the lower region of the left nostril in the last 5 years, which, according to him, were “typical of herpes.” He used topical acyclovir, a medication that is used to lead to almost complete remission of the lesions but no longer did. He then used multiple antimicrobials (including amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and cephalexin) and was prescribed oral and intravenous acyclovir. Despite the various therapeutic strategies, there was a progressive worsening of the lesion that led to the patient’s referral to the dermatology department. On physical examination, there was a warty lesion on the left nasal flap, approximately 7 cm in its largest diameter. It was vegetative, rounded, partially ulcerated, and covered by fibrinous tissue (Figures 1A and 1B). The individual was in good general condition and had no other complaints or systemic alterations worth noting. Once the diagnosis of hypertrophic herpes (HH) was raised, he was again prescribed oral acyclovir, plus topical imiquimod. Despite the new treatment regimen, the lesion continued to progress and increase in size. The patient was then referred to plastic surgery for resection and histopathological analysis of the lesion due to suspicion of an underlying neoplastic process. Complete surgical resection of the lesion was performed, with a safety margin and rotation of the nasolabial musculocutaneous flap. The pathological analysis of the surgical specimen showed that there was “epidermal pseudoepitheliomatous hyperplasia at the edge of an ulcerated lesion, granulation tissue, and exuberant dermal lymphoplasmacytic inflammation (…).” The presence of “cytopathic changes with characteristics of herpes virus (HSV) infection favoring the diagnosis of hypertrophic herpes” was also described. Immunohistochemistry was positive for HSV; in situ hybridization was negative for lowand high-risk papillomavirus (HPV) antigens; and P16 immunohistochemistry in the epithelial cells of interest was negative. In view of these findings, the suspicion of a neoplastic process was excluded and the initial diagnostic hypothesis of HH was confirmed, although refractory to antiviral treatment with acyclovir and topical therapy with imiquimod. Six months after the operation, there was recurrence of the lesion; this time, intravenous foscarnet was prescribed for 10 days, with complete remission. The patient is currently on suppressive antiherpetic therapy with valacyclovir (500 mg/day). He remains under outpatient follow-up with no new recurrences even after 7 months.
Figure 1. Hypertrophic lesion in the left nasal flap due to herpes simplex virus (A - frontal view; B - lateral view).
DISCUSSION
HSV mucocutaneous infections are extremely common, with genital herpes being the most prevalent sexually transmitted infection worldwide1. There are two main biological and antigenic types of HSV: HSV-1, which typically affects the mouth, oral cavity, lips, nostrils, and adjacent skin; and HSV-2, which usually affects the anogenital region2. The prevalence of both HSV types is estimated to comprise approximately 90% of the global population, with HSV-1 being more prevalent than HSV-24. HSV infection is also one of the most common co-infections among patients with HIV, with seroprevalence ranging from 50% to 90% in different regions of the world 5.
Atypical presentations of HSV infection among patients with HIV are frequent. Within the spectrum of atypical forms of the disease, the hypertrophic or pseudotumoral form is considered rare, with just over 100 case reports found in the literature4.
Hypertrophic herpes lesions are usually disfiguring conditions presented as painful exophytic nodular tumors, with or without superficial ulceration that predominantly affect the anogenital region, but can also appear in other sites, such as the mouth and oral cavity, nose, ears, and periocular and conjunctival regions, as well as in rarer sites, such as the larynx, vocal cords, endobronchial mucosa, and hands and feet2,6. Our patient’s lesion was located in the nasal region, a topography with very few cases reported2,7,8. It should be noted that diagnostic and therapeutic strategies and interventions for the management of hypertrophic lesions due to HSV are independent of the anatomical site of involvement, that is, the management of hypertrophic lesions in rarer sites such as the orofacial region does not differ from that of anogenital lesions, for example2,3.
The investigation of a case of HH can be laborious, and other diseases that may present with vegetative verrucous lesions should be ruled out, especially those of neoplastic origin such as squamous cell carcinoma, lymphomas, and other hematopoietic neoplasms. Other infectious causes important for the differential diagnosis include HPV, cytomegalovirus, molluscum contagiosum virus, tuberculosis, atypical mycobacteriosis, secondary syphilis, and fungal infections9,10.
Diagnosis confirmation in the reported case was made by biopsy, whose histological analysis showed cytopathic alterations with immunohistochemistry positive for HSV and negative for HPV. The aforementioned p16 protein analysis raised the possibility of a neoplasm as HPV-associated carcinomas are characterized by overexpression of this marker, which is detected by immunohistochemistry11. Other diagnostic methods for the identification of HSV include cell culture, molecular techniques with nucleic acid amplification, and type-specific serological tests. The latter two have been increasingly used during the last decades and have relatively high sensitivity and specificity, although they are not yet widely available in developing countries11.
The pathophysiological mechanisms involved in the genesis of HH have not yet been completely elucidated, although some hypotheses have been proposed based on the results of clinical, pathological, and laboratory studies on the subject. The starting point of the process is considered to be prolonged cutaneous inflammation that causes hyperproliferation of keratinocytes through the secretion of cytokines. In this context, HIV infection contributes through at least two mechanisms: a greater release of these cytokines that stimulate epidermal growth by HIV-infected dendritic cells and the reduction of the negative feedback of interferon-gamma on epidermal proliferation, because HIV-infected individuals have fewer helper and cytotoxic T cells and consequently a lower release of this specific cytokine2,12. Finally, due to the abundance of eosinophils observed in the lesions, Abbo et al. 13 proposed a relationship between Th2 immune response and cell hypertrophy. This hypothesis is corroborated by the good therapeutic response to imiquimod in cases of HH, as this immunomodulator stimulates the Th1 immune response through the production of interferon-alpha, thus altering the center of the inflammatory response and correcting the local immunopathological process.
The management of hypertrophic herpes lesions is, in general, challenging, and refractoriness to first-line systemic antiviral agents such as acyclovir is common 14, as in the present case. In the systematic review by Sasso et al.2, only half of 81 patients diagnosed with HH responded to treatment with first-line antivirals. The real cause of the lack of therapeutic response in these cases is not exactly known. One of the proposed mechanisms is the reduced or absent penetration of the antiviral into the pseudotumoral tissue; another mechanism is a higher frequency of antiviral-resistant HSV strains in immunosuppressed patients3. Other therapeutic strategies have been used in refractory cases, such as the administration of intravenous foscarnet® and the use of topical imiquimod; surgical removal of the lesion is a last resort option2,3,14.
The surgical management of chronic and severe herpes simplex infections has been described since the 1970s, when there were no effective drug treatments for HSV. Following the availability of acyclovir in 1982, surgical treatment became less desirable, both because of its more invasive nature and because of the description of postoperative recurrences in sites adjacent to the primary lesion14-16. Currently, surgical treatment is reserved for cases refractory to other available therapeutic strategies.
In fact, our patient did not respond well to treatment with antivirals or topical application of imiquimod. Therefore, it was decided to surgically remove the lesion, although this decision was reinforced to eliminate the hypothesis of a neoplastic etiology, which was still under consideration. In the same systematic review by Sasso et al.2, surgical resection was used as part of the treatment in approximately 38% of the patients diagnosed with HH; there is no description of the percentage of patients who had recurrence after resection. Simonsen et al.16 compiled nine cases of patients with perianal HH, seven of whom were treated with surgical resection (with or without concomitant antiviral treatment). Of these, three had recurrent disease, similar to what occurred in our patient’s face.
CONCLUSION
This case illustrates how the diagnosis and treatment of hypertrophic lesions caused by HSV are challenging, considering their similarity to invasive carcinomas and high rates of resistance to first-line antivirals. As reported, therapeutic management of HH often involves the use of systemic antivirals, immunomodulatory therapy, and, in more persistent cases, surgical resection.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72(8):1527-34.
2. Sasso BM, Florence ME, Magalhaes RF, Velho PE, de Souza EM, Cintra ML, et al. Herpes simplex virus mucocutaneous tumoural lesions–systematic review. J Clin Virol. 2020;123:104246. Available at: https://www.sciencedirect.com/science/article/abs/pii/S1386653219302768?via%3Dihub
3. Barde C, Piguet V, Pechère M, Masouyé I, Saurat JH, Wunderli W, et al. Management of resistant mucocutaneous herpes simplex infections in AIDS patients: a clinical and virological challenge. HIV Med. 2011;12(6):367-73.
4. Wald A, Corey L. Persistence in the population: epidemiology, transmission. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editores. Human Herpesviruses: Biology, Therapy and Immunoprophylaxis. Cambridge University Press; 2007. Chapter 36. Available at: https://www.ncbi.nlm.nih.gov/books/NBK47447/
5. Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135(11):1393-7.
6. Chiu CY, Randhawa G, Nada K, Tomczak E, Feinstein A, Hennessey K. A nasal hypertrophic lesion as a presentation of herpes simplex virus. IDCases. 2019;15:e00512. Available at: https://www.sciencedirect.com/science/article/pii/S2214250919300162?via%3Dihub.
7. Strehl JD, Mehlhorn G, Koch MC, Harrer EG, Harrer T, Beckmann MW, et al. HIV-associated hypertrophic herpes simplex genitalis with concomitant early invasive squamous cell carcinoma mimicking advanced genital cancer: case report and literature review. Int J Gynecol Pathol. 2012;31(3):286-93.
8. Cury K, Valin N, Gozlan J, Jacquier I, Boutolleau D, Guegan S, et al. Bipolar Hypertrophic Herpes: An Unusual Presentation of Acyclovir-Resistant Herpes Simplex Type 2 in a HIV-Infected Patient. Sex Transm Dis. 2010; 37(2):126-128
9. 9. Beasley KL, Cooley GE, Kao GF, Lowitt MH, Burnett JW, Aurelian L. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5):860-3.
10. Deza G, Martin-Ezquerra G, Curto-Barredo L, Villar Garcia J, Pujol RM. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42(12):1176-8.
11. Arshad Z, Alturkistani A, Brindley D, Lam C, Foley K, Meinert E. Tools for the diagnosis of herpes simplex virus 1/2: systematic review of studies published between 2012 and 2018. JMIR Public Health Surveill. 2019;5(2):e14216. Available at: https://publichealth.jmir.org/2019/2/e14216/
12. Smith KJ, Skelton III HG, Frissman DM, Angritt P. Verrucous lesions secondary to DNA viruses in patients infected with the human immunodeficiency virus in association with increased factor XIIIa-positive dermal dendritic cells. J Am Acad Dermatol. 1992;27(6):943-50.
13. Abbo L, Vincek V, Dickinson G, Shrestha N, Doblecki S, Haslett PA. Selective defect in plasmacyoid dendritic cell function in a patient with AIDS-associated atypical genital herpes simplex vegetans treated with imiquimod. Clin Infect Dis. 2007;44(3):e25-7.
14. Arinze F, Shaver A, Raffanti S. Surgical excision for recurrent herpes simplex virus 2 (HSV-2) anogenital infection in a patient with human immunodeficiency virus (HIV). Infection. 2017;45:705-7.
15. Shelley WB. Surgical treatment for recurrent herpes simplex. Lancet. 1978; 11;312(8098):1021-2.
16. Simonsen M, Nahas SC, Silva Filho EV, Araújo SE, Kiss DR, Nahas CS. Atypical perianal herpes simplex infection in HIV-positive patients. Clinics. 2008;63:143-6.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















