Varicella-zoster virus transverse myelitis in the absence of skin lesions
DOI:
https://doi.org/10.5935/2764-734X.e20231231Keywords:
Varicella zoster virus infection, Myelitis, Transverse, Zoster sine herpete, Case reportAbstract
Varicella-zoster virus (VZV) myelitis is a rare complication in individuals without immune dysfunction, but relatively frequent in immunocompromised individuals. Neurological changes can be serious and lead to death or sequelae, especially if there is a delay in starting the treatment. We report the clinical case of a human immunodeficiency virus-infected trans woman complaining of urinary retention, paresis, and paresthesia of the lower limbs 10 days after the appearance of allodynia in a thoracic dermatome affected by herpes zoster in the past. There were no skin lesions at all, such as the vesicles and blisters typical of VZV reactivation, and all the complementary tests were unable to establish an unequivocal etiological diagnosis. Even so, empirical treatment with high-dose intravenous acyclovir for 14 days exhibited a satisfactory clinical response, with immediate partial reversal of the neurological deficits.
Downloads
INTRODUCTION
Varicella-zoster virus (VZV) or human herpesvirus 3 is a neurotropic virus that has humans as its only reservoir[1]. Primary infection occurs mainly in childhood and manifests as chicken pox, marked by a difuse vesicular rash. After the condition resolves, the virus becomes latent in the nerve ganglia of the posterior dorsal root[2]. Through dysfunction of cellular immunity, VZV can be reactivated and taken to the skin surface through axonal transport[1], causing skin lesions characterized by the presence of vesicles and blisters in diferent stages of evolution, typically painful and in locations delimited by dermatomes. Less frequently, the virus can also spread through the central nervous system, giving rise to conditions such as meningitis, encephalitis, ventriculitis, radiculitis, and myelitis[2,3,4] as well as neurological manifestations that are very similar to various other etiologies, including noninfectious ones. Here, we present a case where the reactivation of VZV caused transverse myelitis without its usual cutaneous alterations—a phenomenon known as zoster sine herpete[1,3], thus further challenging its diagnosis, which was nevertheless corroborated by the satisfactory response to empirical treatment.
CASE DESCRIPTION
A 53-year-old trans woman came to the emergency room complaining of acute urinary retention and sudden loss of strength in both lower limbs (LL) a day ago. She also reported a squeeze or sharp pain of moderate intensity in the right thoracoabdominal transition for 10 days, which worsened on palpation and partially improved with the use of dipyrone. She had been human immunodefciency virus (HIV)-positive since 1992 and was currently on regular antiretroviral treatment with tenofovir, lamivudine, and atazanavir with ritonavir, maintaining an undetectable viral load and a CD4 count of 437 cells/µl. On admission, she was conscious and oriented. Physical examination revealed allodynia (when a non-noxious stimulus is perceived as painful) on simple touch of the dermatome at T8 level, on the right, without any skin changes. The patient recalled having had “herpes” with vesicular rash in this topography about 10 years ago. The pupils were isochoric and photoreagent, the fundus was normal, the patient was eumetric with normal diadochokinetic performance, and showed no alterations in the cranial nerves. She had grade V strength in the upper limbs, but grade III in the LL, associated with paresthesia and an indiferent plantar cutaneous refex.
The laboratory tests carried out were: treponemal antibody absorption (FTA-ABS) and nontreponemal (VDRL) nonreactive tests for syphilis; negative IgG and IgM antibodies for hepatitis C, HTLV and cytomegalovirus (CMV); reactive anti-HBs and nonreactive anti-HBc, confrming vaccine immunity for the B virus; negative antigen test for Cryptococcus sp and undetected qualitative serum polymerase chain reaction (PCR) for CMV. The stool test for Schistosoma sp was negative.
Contrast-enhanced computed tomography (CT) scans of the skull and spine showed no compressive factors or any other signifcant alterations, while the patient refused to undergo magnetic resonance imaging (MRI) claiming claustrophobia.
A sample of lumbar cerebrospinal fuid was collected for laboratory analysis, and the cellularity was 27 cells/ mm³ (typical lymphocytes: 64%, monocytes: 10% and neutrophils: 26%), glycorrhea of 54mg/dl and protein dosage of 232mg/dl. India ink was negative, as were bacterioscopy and aerobic culture. Molecular biology testing for herpes viruses was negative, with not detectable PCR for all types (1, 2, 3, 4, 5, 6).
Prostate ultrasound was normal, with no obstructive factor. Electroneuromyography of the LL was also normal. After ruling out the main diferential diagnoses and in view of the allodynia coinciding with the dermatome afected by Zoster in the past, the diagnostic hypothesis of myelitis secondary to VZV was prioritized.
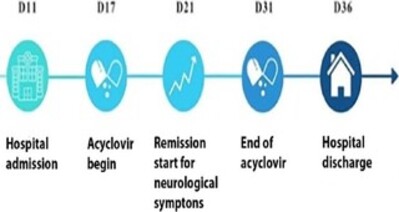
After starting intravenous acyclovir at a dose of 10mg/kg every 8 hours, the patient’s strength improved in the LL within the frst few days, and she was able to stand in an orthostatic position again a week after the treatment’s start. The medication was continued for 14 days, and there was improvement in gait, resolution of pain and sensory alterations; urinary retention, however, continued until discharge, which took place on the 36th day of hospitalization, with the patient having been instructed to perform self-relieving bladder catheterization at home. Although a return visit and outpatient follow-up were scheduled, the patient no longer attended her appointments. Figure 1 illustrates the main facts and events in a timeline for educational purposes.
Figure 1. Timeline in days (D) illustrating the main facts related to the case.
DISCUSSION
Myelopathies can be divided into compressive or infammatory and both have similar signs and symptoms, most frequently including loss of strength and sensitivity below the level of the spinal cord where the injury is located, as well as urinary and/or intestinal retention [2]. Once spinal cord compression has been ruled out (through CT and/or MRI), further investigation should be carried out to identify the specifc infammatory factor, as there are many possible etiologies of myelitis, including infectious, vascular, paraneoplastic, connective tissue diseases, autoimmune, nutritional defciency, poisoning and idiopathic causes [5,6].
Clinically, the presence of fever, meningeal signs and symptoms, skin changes, systemic infection, recent travel history, immunosuppression and adenopathy are some of the factors that may suggest an infectious etiology. In the event of transverse myelitis, these etiologies should be the frst to be considered (especially in immunosuppressed patients), as they represent serious but treatable and potentially reversible causes[6].
Among the infectious causes, the main agents are enterovirus, herpes group virus, HIV, human T-cell leukemia virus type 1 (HTLV-1), Zika virus, West Nile virus, tuberculosis and syphilis[7]; in Brazil, schistosomiasis should be added to this list[5]. It is interesting to note that tuberculosis is also a possible compressive factor (not just inflammatory) and usually affects other organs and systems. Schistosomiasis is associated with the peculiar finding of eosinophils in the cerebrospinal fluid, corresponding to the hepatointestinal form of the disease[5].
In this case, CT ruled out compressive causes and ensured the safe collection of cerebrospinal fluid, the analysis of which showed nonspecific inflammation (through increased cellularity and protein). Microbiological, serological and genetic tests did not indicate a diagnosis, but allowed us to exclude (at least partially) the main suspected etiologies. In this complex scenario, the physical examination showing allodynia restricted to one dermatome, coinciding with a history of herpes zoster, was the main factor justifying the strong suspicion of invasion of the central nervous system by this agent.
The prevalence of neurological complications caused by VZV in the healthy population is low, varying in the literature between 0.1% and 0.3%[8,9]. In immunocompromised patients, however, it can be as high as 35%, especially those with lymphoma, leukemia, metastatic tumors, HIV infection, kidney failure and systemic lupus erythematosus[9]. The typical cutaneous vesicles and blisters present in VZV infections usually anticipate neurological signs and symptoms by around 21 days, and their absence is a rare phenomenon, although certainly underreported[6,10]. There is no relationship between Zoster Sine Herpete and immunosuppression, and this was not the case for our patient, who was adherent to antiretroviral therapy.
In any case, the motor symptoms generally start unilaterally, in a topography adjacent to the level of the affected dermatome, soon becoming bilateral in most cases. This is followed by spinothalamic and posterior column sensory symptoms, as well as urinary or intestinal retention[2]. Post-mortem histopathological studies have shown that the pathogenesis of neurological lesions seems to be directly related to viral invasion and consequent axonal degeneration[4], and the spinal cord can be affected throughout its entire length (although the thoracic region is the most frequent)[5].
The diagnosis of transverse myelitis is made essentially based on clinical history, physical examination, imaging tests, laboratory tests and cerebrospinal fluid analysis. Imaging methods generally do not show specific alterations, the most common being an intramedullary signal in localized topography, seen on magnetic resonance imaging (2). Serological tests take between 7 and 14 days to be positive and are difficult to interpret, since the IgG Index must be used to analyze intrathecal antibody production; and serologies, as usual, lose sensitivity in immunocompromised patients[2,8]. CSF analysis most commonly shows nonspecific alterations such as lymphomonocytic pleocytosis and/or elevated protein levels, but it also allows research into antibodies against VZV and the detection of viral DNA by PCR[2]. In fact, PCR techniques are highly specific, but only in the acute phase of the infection, within the first 7 to 10 days of the onset of the rash. In this phase, the sensitivity of the test varies between 60% and 100% and can drop to less than 25% after this period - a fact that may justify the negative PCR in our patient, as the test was collected after the 10th day of the onset of symptoms and there were no skin lesions, a factor that has already been pointed out as a risk factor for failure to detect viral DNA[8]. It can therefore be seen that complementary tests are often not enough to unequivocally confirm central involvement by VZV, but in a suspicious clinical context, this hypothesis should never be excluded, even if there is no detection of specific DNA or IgG [2,11].
We found no clinical trials in the literature capable of establishing a standardized treatment regimen for VZV myelitis. However, the postmortem histopathological finding of active viral infection in neuronal tissues[4] has been the main argument to justify the use of high-dose antiviral therapy. Acyclovir is the most widely used antiviral, although valacyclovir and famciclovir can be used as alternative treatments[8]. Following the same logic, evidence of vasculitis in the tissues of patients with neurological sequelae has been used to justify the concomitant use of corticosteroids. Several case reports on the use of combined therapy have been published with satisfactory results, but there is still not enough evidence to support this recommendation[2].
In our case, the patient was treated with only acyclovir (without corticosteroids) and progressed with a satisfactory clinical response and partial reversal of symptoms just a few days after starting therapy. It is worth remembering that, despite treatment (whether combined or not), the rate of sequelae in these patients is high and factors such as the rapid progression of neurological deficits, immunosuppression, paraplegia and urinary retention seem to be associated with greater morbidity. Early diagnosis and treatment, on the other hand, is the most favorable prognostic factor [2].
CONCLUSION
This report illustrates the importance of always considering invasion of the central nervous system by VZV as a diagnostic hypothesis for cases of myelitis, especially in patients with immune dysfunction and even in the absence of specifc biochemical, radiological, serological and genetic tests - even in the absence of skin lesions. Although the treatment in this case was empirical (motivated by allodynia and careful clinical reasoning), it was introduced relatively early and led to clear therapeutic success in the short term.
References
1. Moshayedi P, Thomas D, Rinaldo CR, Moossy JJ, Maroon JC, Murdoch GH, et al. Subacute histopathological features in a case of varicella zoster virus myelitis and post-herpetic neuralgia. Spinal Cord Ser Cases. 2018 Apr;4(1):33. DOI: 10.1038/s41394-018-0068-5
2. Lameiras C, Patrocínio de Jesus R, Flor-de-Lima B, Silva J, Pacheco P. A case of varicella-zoster virus meningomyelitis in an HIV-1-infected patient: facing the challenges related to its management and prognosis. Cureus. 2022 Aug;14(8):e27652. DOI: 10.7759/cureus.27652
3. Liu Q, Zhou X, Li Z. Acute myelitis with multicranial neuritis caused by varicella zoster virus: a case report. BMC Neurol. 2022;22(1):45. DOI: 10.1186/s12883-022-02571-y
4. Yun D, Cho SY, Ju W, Seo EH. Transverse myelitis after infection with varicella zoster virus in patient with normal immunity: a case report. World J Clin Cases. 2021 Nov;9(33):10308-14. DOI: 10.12998/wjcc.v9.i33.10308
5. Cree BAC. Acute inflammatory myelopathies. Handb Clin Neurol. 2014;122:613-67. DOI: 10.1016/B978-0-444-52001-2.00027-3
6. Simone CG, Emmady PD. Transverse Myelitis. [Updated 2022 Nov 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Disponível em: https://www.ncbi.nlm.nih.gov/books/NBK559302/
7. West TW. Transverse myelitis - a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-77. Disponível em: https://pubmed.ncbi.nlm.nih.gov/24099672/
8. Miyake Z, Tomidokoro Y, Nohara S, Tamaoka A. Chronic myelitis associated with zoster sine herpete. A case report. Medicine. 2019 Aug;98(32):e16671. DOI: 0.1097/MD.0000000000016671
9. Blanchardiere AD, Rozenberg F, Caumes E, O Picard, F Lionnet, J Livartowski, et al. Neurological complications of varicella-zoster virus infection in adults with human immunodeficiency virus infection. Scand J Dis. 2000;32(3):263-9. DOI: 10.1080/00365540050165893
10. Zhou J, Li J, Ma L, Cao S. Zoster sine herpete: a review. Korean J Pain. 2020 Jul;33(3):208-15. DOI: 10.3344/kjp.2020.33.3.208
11. Takahashi T, Tamura M, Miki K, Yamaguchi M, Kanno A, Nunomura S, et al. Varicella zoster virus myelitis in two elderly patients: diagnostic value of nested polymerase chain reaction assay and antibody index for cerebrospinal fluid specimens. Case Rep Neurol. 2013 Apr;5(1):81-90. DOI: 10.1159/000350714
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















