Mycobacterium mucogenicum related to granulomatosis with polyangiitis: infection or colonization?
DOI:
https://doi.org/10.5935/2764-734X.e20231233Keywords:
Non-tuberculous mycobacteria, Granulomatosis with polyangiitis, Vasculitis, Molecular Biology, Case ReportAbstract
Advances in diagnostic methods, especially molecular biology, have made the identification of microorganisms easier and more sensitive. However, this progress also requires a better assessment of the clinical significance of the isolated microorganism, since in many situations this agent may only be contaminant or tissue colonization. Non-tuberculous mycobacteria are good examples of this duality and can cause both a range of clinical infections or only colonize certain sites in the body. In this report, we describe the case of a previously healthy patient with ulcerated lesions of the skin and mucous membranes with a chronic and progressive evolution. After various tests and evaluations, granulomatosis with polyangiitis was diagnosed. However, the biopsy of the nasolabial lesion also identified the presence of Mycobacterium mucogenicum using molecular biology. This identification raised many doubts in the medical teams about the real role of this Non-tuberculous mycobacteria in the pathogenesis or aggravation of the lesions, with decision-making implications about its specific treatment (or not), since the patient would be undergoing immunosuppressive therapy.
Downloads
INTRODUCTION
The Mycobacterium mucogenicum group includes M. mucogenicum, M. aubagnense, and M. phocaicum, all considered to be fast-growing mycobacteria. This group of microorganisms was initially named Mycobacterium chelonae-like in 1982, when it was reported as the etiologic agent of an outbreak of peritonitis involving two peritoneal dialysis units1.
Infection with M. mucogenicum is rare. When identified, most of the time it is colonizing a tissue or is a finding of no clinical relevance2. Individuals undergoing hemodialysis, however, as well as those who have recently undergone surgical procedures and hospitalizations, seem to be at greater risk of being colonized (or infected) by this mycobacterium, since this microorganism is present in hospital water systems3,4. M. mucogenicum has an important capacity for biofilm formation, which allows it to tolerate extreme temperatures and various types of disinfection5.
When the presence of M. mucogenicum represents active infection, it can be associated with a broad spectrum of clinical manifestations. There are reports in the literature of respiratory conditions and involvement of the skin and soft tissues, without much epidemiological relevance6. Catheter-related bloodstream infections, on the other hand, are the most common - especially long-term catheters7 in immunosuppressed patients (cancer patients and bone marrow transplant patients)8. It is believed that contamination can occur during bathing, when catheters are exposed to water from the shower9.
In this case report, the presence of Mycobacterium mucogenicum was identified using molecular biology in a biopsy of an ulcerated lesion in the nasolabial region of a patient with granulomatosis with polyangiitis (GPA), an autoimmune disease. A discussion begun about the role of the mycobacterium in this context, as well as whether we should consider treating it, given that the patient would still be undergoing immunosuppressive therapy.
CASE REPORT
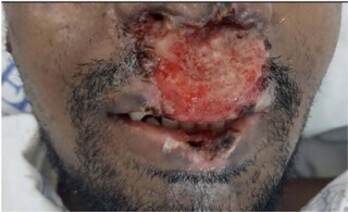
A 30-year-old man was admitted to a tertiary referral hospital for an investigation of a chronic lesion causing significant nose deformity, with perforation of the septum and collapse of the nasal dorsum. There were also some systemic symptoms, such as weight loss (around 10 kg in the last year), asthenia and fatigue. The patient was previously healthy, with no relevant pathological or epidemiological history, born in the Chapada Diamantina region of Bahia, Brazil, but had lived in São Paulo, Brazil, since he was 14 years old. In his anamnesis, he reported frequent episodes of epistaxis and sinusitis for more than 10 years, always treated with oral antibiotics, but without complete resolution of the condition. About two years ago, he noticed the appearance of small ulcerated and crusted lesions in the nasolabial region, which, at the beginning of the condition, disappeared spontaneously. In the last six months, these lesions have grown and agglutinated into a single larger lesion, also affecting the nasal mucosa (Figure 1A). He also mentioned that he had already received medical care at various hospitals and clinics and had even undergone biopsies and procedures such as nasofibroscopy - all of which were inconclusive, ratified by reports provided by the patient himself.
Figure 1A. Ulcerated lesion (pre-treatment) in the nasolabial region, when the patient was admitted to hospital to begin investigation.
The main diagnostic hypothesis at the beginning of the hospitalization was mucocutaneous leishmaniasis, but the serology was negative, as was the sorology of paracoccidioidomycosis (by immunoblot). Other serological tests were also negative: anti-hepatitis C, antihepatitis B, VDRL and anti-HIV. Other laboratory tests showed high platelet count (758 thousand/mm3), anemia (hemoglobinemia of 8.4 g/dL) and increased C-reactive protein (CRP) (35.1 mg/L compared to the reference value of up to 10 mg/L). Computed tomography scans of the sinuses showed points of mucous thickening distributed throughout the paranasal cavities bilaterally, with protein content in the left maxillary sinus. Part of the hard palate, the nasal septum, the middle and lower nasal turbinate and the uncinate processes were not characterized (Figure 2).
Figure 2. CT scan of the sinuses showing mucous thickening distributed throughout the paranasal cavities bilaterally, with protein content in the left maxillary sinus. Part of the hard palate, the nasal septum, the middle and lower nasal turbinate and the uncinate processes were not characterized.
A new biopsy of the nasolabial lesion was carried out, which histological analysis (hematoxylin and eosin, Zielh-Neelsen, Grocott and PAS staining) revealed a “chronic ulcer with acute suppurative component and atypical cellular infiltrate in the skin integument”. Histochemical tests were negative for acid-fast bacteria (AFB) and fungi. All cultures for bacteria, fungi and mycobacteria from the blood and the biopsied fragment were negative. The investigation of leishmania was again negative, this time by polymerase chain reaction (PCR). The only agent detected, also by molecular biology (PCR), was Mycobacterium spp, whose sequencing showed 100% nucleotide similarity with M. mucogenicum.
In view of the controversy surrounding the role of this mycobacterium as the causative agent of such tissue destruction, we decided to involve the rheumatology team in the multidisciplinary discussion. These experts requested several autoimmunity markers, whereas c-ANCA was reactive (with a titration of 1/60), as was anti-proteinase 3 (anti-PR3). These results led to the diagnosis of granulomatosis with polyangiitis (GPA), whose proposed treatment was pulse therapy (methylprednisolone 1000 mg daily for three days) combined with methotrexate 15 mg/week. Even in view of the immunosuppression which the patient would be subjected, the teams involved opted to not treat the mycobacterium: in addition to the c-ANCA and anti-PR3 markers being highly specific for the diagnosis of GPA, the patient’s clinical condition (epistaxis and recurrent sinusitis preceding the appearance of the nasolabial lesion) could already be explained by the underlying disease alone, with no evidence of an associated infection. M. mucogenicum, therefore was colonizing a chronic wound that already had an ulcerated surface for months, and previous procedures and manipulations in a hospital environment may have been a risk factor in facilitating local colonization by this microorganism.
One year after diagnosis and the start of treatment, the patient is in good condition. There was total regression and healing of the ulcerated lesions, as well as improvement in the systemic condition, with weight gain and reduced fatigue. There were no clinical manifestations suggestive of invasive or active mycobacteriosis, nor was there any worsening or the appearance of new wounds after the introduction of immunosuppressive treatment (Figure 1B).
Figure 1B. Regression of the lesion after specific treatment (after one year).
DISCUSSION
According to the growth time of their colonies on culture media, non-tuberculous mycobacteria (NTMs) are divided into two groups: the fast-growing ones, which produce mature colonies on agar plates within seven days; and the slow-growing mycobacteria, which take longer than seven days to grow. The M. mucogenicum identified in the case reported belongs to the first group10.
The ecological niche of M. mucogenicum is poorly known, but this species seems to be ubiquitous and mainly colonizes water systems of communities and hospitals5,9. This is why most infections caused by this pathogen are related to recent hospitalization or association with a contaminated water source.
Infections caused by NTMs have been increasingly reported in recent years, possibly due to the increase in medical conditions and treatments that compromise the immune system, such as the use of immunobiologicals, chemotherapy and transplants11, but also due to the improvement and greater availability of the techniques needed to identify it. The current gold standard for identifying mycobacteria is DNA sequencing with the 16sRNA, rpoB and hsp65 genes being recognized as useful targets, but these are not easily accessible methods in many laboratories. Mass spectrometry (MALDI-TOF) can also accurately identify species of mycobacteria12.
Despite the greater possibility that it is just contamination, the identification of NTMs in the bloodstream or in some other location that is related to the patient’s symptoms should be considered a situation that requires treatment, especially in immunocompromised individuals. It is therefore important to remember that if a NTM is identified and there is some clinical relevance, antimicrobial susceptibility testing should be arranged. In our case, there was no growth of M. mucogenicum in culture that would allow us to obtain this information.
Isolates of NTMs are usually susceptible to aminoglycosides, fluoroquinolones, tetracyclines, macrolides, carbapenems, cefoxitin, sulfamethoxazoletrimethoprim and linezolid. The ideal antibiotic therapy is not standardized, and many treatment regimens have been proposed. According to the consensus of the Infectious Diseases Society of America, an aminoglycoside combined with a macrolide and/or a quinolone is the most appropriate empirical treatment. The ideal duration of treatment is unknown, but at least four weeks of treatment is recommended, which can be extended between 12 and 18 months13.
In this case, it was concluded that the isolated NTM did not participate in the pathogenesis of the disease. In addition to the fact that it did not grow in culture, we did not find any reports in the literature where M. mucogenicum had caused this type of severe, deforming lesion, and the underlying disease - GPA - was already capable of justifying the entire clinical picture presented by the patient14. In addition, there was no recommendation for preemptive treatment for this pathogen or any other NTM, despite the immunosuppression to which the patient would be subjected to treat the GPA. The decision of the multidisciplinary team was therefore to maintain an expectant attitude, with the necessary supervision and monitoring to immediately identify any clinical and harmful manifestations that might be related to the mycobacterium.
CONCLUSION
The presence of M. mucogenicum in human tissues raises doubts about its pathogenicity and clinical relevance, which makes decision-making difficult when this pathogen is identified, especially in immunosuppressed patients. In the case reported, this fast-growing mycobacterium was considered to be colonizing the tissue and was not related to the development of the lesion, since the patient had a diagnosis that explained by itself the evolution and all the signs and symptoms presented. The decision to not treat this mycobacterium proved to be the right one, because even in the face of immunosuppressive treatment for the underlying disease, there was no clinical worsening or any evidence of NTM-related activity.
References
1. Band JD, Ward JI, Fraser DW, Peterson NJ, Silcox VA, Good RC, et al. Peritonitis due to a mycobacterium chelonei-like organism associated with intermittent chronic peritoneal dialysis. J Infect Dis. 1982 Jan;145(1):9-17. DOI: 10.1093/infdis/145.1.9
2. Esteban J, Martín-de-Hijas NZ, Fernandez AI, Fernandez-Roblas R, Gadea I; Madrid Study Group of Mycobacteria. Epidemiology of infections due to nonpigmented rapidly growing mycobacteria diagnosed in an urban area. Eur J Clin Microbiol Infect Dis. 2008 May;27(10):951-7. DOI: 10.1007/s10096-008-0521-7
3. Livni G, Yaniv I, Samra Z, Kaufman L, Solter E, Ashkenazi S, et al. Outbreak of Mycobacterium mucogenicum bacteraemia due to contaminated water supply in a paediatric haematology-oncology department. J Hosp Infect. 2008 Nov;70(3):253-8. DOI: 10.1016/j.jhin.2008.07.016
4. Adékambi T. Mycobacterium mucogenicum group infections: a review. Clin Microbiol Infect. 2009 Oct;15(10):911-8. DOI: 10.1111/j.1469-0691.2009.03028.x
5. Simões LC, Simões M, Vieira MJ. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol. 2007 Oct;73(19):6192-200. DOI: 10.1128/AEM.00837-07
6. Han XY, Dé I, Jacobson KL. Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases. Am J Clin Pathol. 2007 Jan;128(4):612-21. DOI: 10.1309/1KB2GKYT1BUEYLB5
7. Hawkins C, Qi C, Warren J, Stosor V. Catheter-related bloodstream infections caused by rapidly growing nontuberculous mycobacteria: a case series including rare species. Diagn Microbiol Infect Dis. 2008 Jun;61(2):187-91. DOI: 10.1016/j.diagmicrobio.2008.01.004
8. Gaviria JM, Garcia PJ, Garrido SM, Corey L, Boeckh M. Nontuberculous mycobacterial infections in hematopoietic stem cell transplant recipients: characteristics of respiratory and catheter-related infections. Biol Blood Marrow Transplant. 2000;6(4):361-9. DOI: 10.1016/s1083-8791(00)70012-7
9. Kline S, Cameron S, Streifel A, Yakrus MA, Kairis F, Peacock K, et al. An outbreak of bacteremias associated with Mycobacterium mucogenicum in a hospital water supply. Infect Control Hosp Epidemiol. 2004;25(12):1042-9. DOI: 10.1086/502341
10. Behra PRK, Pettersson BMF, Ramesh M, Dasgupta S, Kirsebom LA. Insight into the biology of Mycobacterium mucogenicum and Mycobacterium neoaurum clade members. Sci Rep. 2019 Dec;9(1):19259. DOI: 10.1038/s41598-019-55464-5
11. Redelman-Sidi G, Sepkowitz KA. Rapidly growing mycobacteria infection in patients with cancer. Clin Infect Dis. 2010 Aug;51(4):422-34. DOI: 10.1086/655140
12. Kodana M, Tarumoto N, Kawamura T, Saito T, Ohno H, Maesaki S, et al. Utility of the MALDI-TOF MS method to identify nontuberculous mycobacteria. J Infect Chemother. 2016 Jan;22(1):32-5. DOI: 10.1016/j.jiac.2015.09.006
13. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. DOI: 10.1164/rccm.200604-571ST
14. Maz M, Chung SA, Abril A, Langford CA, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of giant cell arteritis and Takayasu arteritis. Arthritis Rheumatol. 2021 Jul;73(8):1349-65. DOI: 10.1002/art.41774
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















