Rhabdomyolysis secondary to influenza virus infection
DOI:
https://doi.org/10.5935/2764-734X.e20240139Keywords:
Influenza Human, Rhabdomyolysis, Influenza A Virus, H1N1 Subtype, Creatine Kinase, Case ReportAbstract
Rhabdomyolysis is a potentially serious condition that may result from influenza infection. We report the case of a 32-year-old man with no comorbidities who presented with fever, myalgia, and dark urine that had been developing for 4 days, followed by cough and runny nose. The tests showed a significant increase in creatine phosphokinase (64,617 U/L) and transaminases. Before specific confirmation of Influenza A and B infection, the main diagnostic hypotheses to justify the condition were infectious etiology, particularly leptospirosis and acute viral hepatitis. Apart from ceftriaxone medication for 10 days, the patient received early vigorous intravenous hydration that contributed to favorable progression of the clinical presentation and laboratory results and to the absence of kidney damage.
Downloads
INTRODUCTION
Rhabdomyolysis is a medical condition that occurs when destroyed or damaged skeletal muscle tissue breaks down, releasing intracellular components into the bloodstream. This condition is clinically characterized by myalgia, muscle weakness, and pigmenturia, with laboratory tests showing an increase in intracellular substances, namely creatine phosphokinase (CPK), myoglobin, electrolytes, lactate dehydrogenase, and aspartate aminotransferase (AST), released by the destroyed myocyte1. Depending on the severity, rhabdomyolysis can progress potentially life-threatening conditions, such as acute renal failure, hydroelectrolytic disorders, and disseminated intravascular coagulation. Early diagnosis of the condition and immediate hydration reduces acute kidney injury1.
Rhabdomyolysis can have traumatic and nontraumatic (e.g., infections) causes1,2. Influenza virus is the main viral etiology among the nontraumatic causes3. Given the seasonality of influenza and its rapid spread during outbreaks, respiratory complications caused by this virus are common in medical care4,5. However, it is possible that many of its extrapulmonary complications have not been properly explained and recognized.
We present the case of a young man with rhabdomyolysis secondary to influenza infection. Although this patient had a benign evolution, this is a potentially serious complication that should be kept in mind and anticipated; moreover, this viral etiology should be especially considered when rhabdomyolysis is associated with fever and flu-like symptoms.
CASE REPORT
A 32-year-old man with no comorbidities presented with fever, generalized myalgia, and dark urine that had been developing for 4 days. He reported muscle weakness, mild headache, and hyporexia. He did not mention any symptoms of jaundice, rash, or skin problems, as well as diarrhea, abdominal pain, nausea, or hemorrhage. There was no history of trauma, strenuous physical exercise, use of drugs, and consumption of anabolic steroids or herbal medicines. Additionally, he denied direct contact with animals, floodwaters, or ticks.
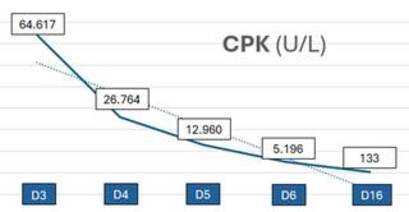
On day 3 with symptoms, he went to the emergency department, where a CPK test was performed due to his complaints. He was given intravenous hydration with crystalloids and discharged the same day, without the CPK result. On the following day the appearance of urine improved, but the fever, myalgia, and malaise persisted. He started coughing yellowish sputum, hyaline rhinorrhea, and sneezing, but did not experience dyspnea or respiratory discomfort. He was admitted to hospital with a fever (38.2°C), normal blood pressure (123 × 83 mmHg), heart rate of 89 bpm, and respiratory rate of 17 bpm. He was in good general condition and had no other findings on physical examination. At this point, the result of the previous serum CPK test revealed 64,617 U/L. Laboratory tests were repeated (Table 1) showing slightly less elevated serum CPK level, a significant increase in transaminases, especially AST, and no renal dysfunction. The initial diagnostic hypotheses were leptospirosis, hepatitis A, and dengue fever given the epidemiological context (a month with rain and flooding in the city), the increase in CPK, the darkened color of the urine, and the acute hepatitis associated with fever. In addition to increased intravenous hydration with crystalloids, ceftriaxone was administered to the patient because of the higher suspicion of leptospirosis.
| Laboratory tests | D3 | D4 | D5 | D6 | D16 |
|---|---|---|---|---|---|
| CPK (U/L) | 64.617 | 26.764 | 12.960 | 5.196 | 133 |
| ALT (U/L) | - | 375 | 297 | 253 | 101 |
| AST (U/L) | - | 1035 | 697 | 406 | 50 |
| Alkaline phosphatase (U/L) | - | 85 | 80 | - | 93 |
| Gamma-glutamyl transferase (U/L) | - | 27 | 26 | - | 39 |
| Total bilirubin (mg/dL) | - | 0,40 | - | - | 0,40 |
| Hemoglobin (g/dL) | - | 13,2 | 12,2 | 13,0 | 12,4 |
| Hematocrit (%) | - | 39,2 | 36,4 | 39,6 | 35,7 |
| Total leukocytes (thousand/mm3) | - | 4800 | 4100 | 4000 | 5400 |
| Neutrophils (thousand/mm3) | - | 3200 | 2400 | 2000 | 2900 |
| Lymphocytes (thousand/mm3) | - | 1200 | 1200 | 1400 | 1800 |
| Platelets (thousand/mm3) | - | 248.000 | 220.000 | 263.000 | 364.000 |
| Creatinine (mg/dL) | - | 0,90 | 0,70 | 0,70 | 0,80 |
| Urea (mg/dL) | - | 32 | 20 | 28 | 33 |
| Sodium (mmol/L) | - | 141 | 137 | 137 | 142 |
| Potassium (mmol/L) | - | 4,1 | 4,2 | 4,9 | 4,7 |
| C-reactive protein (mg/L) | - | 7,60 | - | 14 | - |
The patient had a good clinical evolution, fever resolved and myalgia improved in just over 48 h, as well as a significant and rapid improvement in the laboratory test findings (Table 1). The hepatitis A, hepatitis C, syphilis, and HIV serologies were nonreactive; the hepatitis B serology showed vaccine immunity. Immunochromatography for dengue to detect the NS1 antigen was also non-reactive. Due to flu-like symptoms (runny nose, coughing, and sneezing), rapid nasopharyngeal swab tests were carried out for covid-19 antigen (negative) and influenza virus (positive for types A and B). However, it was decided to continue the antibiotic treatment due to the remaining suspicion of leptospirosis and its potential severity. The patient was discharged on the third day of hospitalization (the seventh day since the onset of symptoms) with a prescription for doxycycline to complete the antibiotic therapy regimen and was instructed to return for reassessment 10 days later. On returning to the outpatient clinic, the patient was asymptomatic and practically all the tests were normal (Table 1). The results of the pending serologies were as follows: IgM was not reactive for leptospirosis, toxoplasmosis, cytomegalovirus (CMV), and Epstein-Barr virus (EBV); IgG was reactive only for CMV and EBV. Therefore, clinically and laboratory-confirmed rhabdomyolysis without any major consequences could be concluded to be secondary to confirmed influenza virus infection.
DISCUSSION
The influenza virus belongs to the Orthomyxoviridae family and its genome consists of negative-sense single-strand RNA4,5. The behavior of the virus is epidemic and sporadically pandemic, with seasonal and interpandemic forms caused by influenza A and B and pandemic forms caused by influenza A4. In the last century, there have been four influenza pandemics, namely in the years 1918, 1957, 1968, and the last one in 2009 (influenza A H1N1)4,5. Vaccination is one of the most effective measures for preventing and controlling influenza infection4.
This case occurred in the city of Sao Paulo, a couple of years ago, in September. In Brazil, the seasonality of the disease is less defined compared to temperate countries6: there are influenza cases throughout the year, although a greater number of cases occur in autumn and winter7. Since 1999, the Brazilian public immunization program included annual influenza vaccination for populations at higher risk of disease exposure7. Since this patient was not part of this target population, he did not remember the last time he had been vaccinated, but he was sure that he had not been vaccinated in the current year.
Influenza infection leads to acute respiratory disease with a variable spectrum of severity, ranging from mild disease limited to the upper respiratory tract to severe disease with extensive pneumonia, secondary bacterial pneumonia, exacerbation of any underlying respiratory disease and non-pulmonary complications5. Nonpulmonary complications include myocarditis, pericarditis, encephalitis, aseptic meningitis, Guillain-Barré syndrome, myositis, and rhabdomyolysis2,4.
The clinical triad of rhabdomyolysis comprises myalgia, muscle weakness, and pigmenturia1. CPK is the gold standard marker for rhabdomyolysis in laboratory testing. There is no well-defined cut-off value but it is usually considered to be five times the upper limit of normal, and concentrations greater than 5,000 U/L are associated with kidney damage1. AST may be elevated and should lead to suspicion of rhabdomyolysis when associated with muscle symptoms1. Herein, the patient presented to us with the classical triad of symptoms and fever. The CPK level was 64,617 U/L on the third day of symptoms, and an elevated AST of 1,035 U/L drew the attention of the medical team on day 4 of symptoms.
Apart from trauma, rhabdomyolysis can be caused by medication, alcohol consumption, drugs abuse, metabolic and electrolyte disorders, muscle ischemia, convulsions, genetic syndromes, and infections1. Anamnesis excluded medications’ use, drugs abuse, anabolic steroids, as well as a history of trauma or strenuous physical exercise in the days preceding the symptoms. Having ruled out other causes and considering the clinical and laboratory relevance of rhabdomyolysis plus fever and flu-like symptoms (cough, runny nose, and sneezing), infection became the main hypothesis.
Rhabdomyolysis can be triggered by bacterial, fungal, and viral infections1,2. The proposed mechanisms involve localized muscle disease, tissue hypoxia secondary to sepsis and dehydration, release of toxins, an immunological reaction through the release of cytokines, fever, tremors, and direct pathogenic invasion into muscle tissue1,2. In the case of influenza, the surface glycoproteins-hemagglutinin and neuraminidase-are the basis of the antigenic properties and pathophysiological mechanism of this infection4. Hemagglutinin uses the sialic acid present in the susceptible host cell as a receptor for attachment, adhesion, and fusion of the viral envelope with the cell membrane4. Thus, viral tropism for host tissues is partly determined by the specificity of the viral surface glycoproteins for sialic acid, which acts as a receptor on the cell8. In a study on the influenza A H1N1 variant and muscle cells, the presence of sialic acids related to viral invasion was observed in human myotubes and myoblasts, making these cells highly susceptible to Influenza infection9. During in vitro influenza infection, there was intracellular viral replication that led to cell lysis and the release of virions into the tissue9. The occurrence of rhabdomyolysis depends on the extent of the damage and break down of skeletal muscle tissue1,10, which reinforces the hypothesis that the mechanism by which influenza causes rhabdomyolysis is the direct invasion of cells by the virus9.
A large part of the evidence of rhabdomyolysis in influenza infection comes from studies and observations carried out since 2009 due to the influenza A (H1N1) pandemic. Although we did not find the estimated incidence, a series of 18 cases showed that 62% of the patients evaluated had CPK levels above 240 IU/L, with five of them having CPK above 1000 IU/L11. In the context of this pandemic, an observational study with 505 patients admitted to the ICU with severe acute respiratory syndrome and H1N1 suggested CPK as a biomarker of severe infection12. Higher CPK levels at the time of ICU admission were associated with acute kidney injury, dialysis, mechanical ventilation, and longer ICU stay12. In this study, 23% of patients had CPK >500 IU/L on admission12. Some authors believe that, in patients hospitalized for influenza, CPK measurement is essential to detect rhabdomyolysis early and initiate measures to prevent acute kidney injury13. Vigorous hydration is the main measure to avoid this complication1.
Although the increase in CPK level was not initially linked to the swab positive for influenza in the present case, the literature indicates that this specific infection is the most frequent viral etiology of rhabdomyolysis3,10. A review of 42 cases of viral rhabdomyolysis showed influenza as the etiology in 33% of cases, followed by Coxsackie virus in 16%10. In this same review, eight of the 14 patients with Influenza rhabdomyolysis had acute kidney injury10. Another study analyzed a series of cases of rhabdomyolysis of bacterial and viral etiology in terms of the relationship between the etiological agent and the corresponding clinical evolution3: influenza was the most associated virus, corresponding to 42% of the 59 cases, followed by Coxsackie virus and HIV, each accounting for 13% of the cases3. Of the 25 patients with influenza, 11 (44%) had acute renal failure and three (12%) died3. In our case, despite high CPK levels and pigmenturia due to myoglobin, the patient did not develop kidney damage.
There are some literature reports concerning the use of specific antiviral therapy with oseltamivir in patients with influenza-induced rhabdomyolysis2,13-15. There are no specific recommendations regarding its use in viral rhabdomyolysis, but the drug is indicated for hospitalized patients and can be beneficial by reducing viral proliferation and, consequently, the length of illness16. In this context, antiviral therapy would have been indicated at the time of hospitalization in the case reported herein. However, the absence of comorbidities, the delay in the correlation between symptoms and viral etiology and, above all, the satisfactory clinical evolution since admission were the reasons why this medication was not prescribed.
CONCLUSION
Early identification of rhabdomyolysis and vigorous hydration are crucial to the favorable clinical outcome and prevention of acute kidney injury described in this report. Therefore, rhabdomyolysis is a potentially life-threatening condition that can arise as a complication of viral infections, particularly influenza. It is important to identify its potential systemic involvement (beyond the well-known respiratory complications) and its atypical manifestations, especially in view of the large number of people infected by this virus every year.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4365849/
2. Fadila MF, Wool KJ. Rhabdomyolysis secondary to influenza a infection: a case report and review of the literature. N Am J Med Sci. 2015;7(3):122-4. DOI: https://doi.org/10.4103/1947-2714.153926
3. Singh U, Scheld WM. Infectious etiologies of rhabdomyolysis: three case reports and review. Clin Infect Dis. 1996;22(4):642-9. DOI: 10.1093/clinids/22.4.642
4. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708. DOI: 10.1016/S0140-6736(17)30129-0
5. Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3. DOI: 10.1038/s41572-018-0002-y
6. Almeida ARM. Dinâmica sazonal da influenza no Brasil: a importância da latitude e do clima [Tese na Internet]. Rio de Janeiro: Escola Nacional de Saúde Pública Sergio Arouca (ENSP)/Fundação Oswaldo Cruz (FIOCRUZ); 2018; [acesso em 2023 Out 10]. Disponível em: https://www.arca.fiocruz.br/bitstream/handle/icict/34080/ve_Alexandra_Ribeiro_ENSP_2018?sequence=2&isAllowed=y
7. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância em Saúde e Ambiente. Informe Técnico Operacional: Vacinação contra a Influenza [Internet]. 1ª ed. Brasília: Ministério da Saúde; 2023; [acesso em 2024 Jan 31]. Disponível em: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/c/cale8ndario-nacional-de-vacinacao/arquivos/informe-tecnico-operacional-de-vacinacao-contra-a-influenza-2023
8. Nicholls JM, Chan RW, Russell RJ, Air GM, Peiris JS. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 2008;16(4):149-57. DOI: 10.1016/j.tim.2008.01.008
9. Desdouits M, Munier S, Prevost MC, Jeannin P, Butler-Browne G, Ozden S, et al. Productive infection of human skeletal muscle cells by pandemic and seasonal influenza A(H1N1) viruses. PLoS One. 2013;8(11):e79628. DOI: 10.1371/journal.pone.0079628
10. Tanaka T, Takada T, Takagi D, Takeyama N, Kitazawa Y. Acute renal failure due to rhabdomyolysis associated with echovirus 9 infection: a case report and review of literature. Jpn J Med. 1989;28(2):237-42. DOI: 10.2169/internalmedicine1962.28.237
11. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680-9. DOI: 10.1056/NEJMoa0904252
12. Borgatta B, Pérez M, Rello J, Vidaur L, Lorente L, Socías L, et al. Elevation of creatine kinase is associated with worse outcomes in 2009 pH1N1 influenza A infection. Intensive Care Med. 2012;38(7):1152-61. DOI: 10.1007/s00134-012-2565-5
13. Runnstrom M, Ebied AM, Khoury AP, Reddy R. Influenza-induced rhabdomyolysis. BMJ Case Rep. 2018;11(1):e226610. DOI: 10.1136/bcr-2018-226610
14. Sato E, Nakamura T, Koide H. Rhabdomyolysis induced by influenza A infection: case report and review of literature. Ther Apher Dial. 2011;15(2):208-9. DOI: 10.1111/j.1744-9987.2010.00900.x
15. Lai CC, Wang CY, Lin HI. Rhabdomyolysis and acute kidney injury associated with 2009 pandemic influenza A(H1N1). Am J Kidney Dis. 2010;55(3):615. DOI: 10.1053/j.ajkd.2010.01.002
16. Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, et al. clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal Influenza. Clin Infect Dis. 2019;68(6):895-902. DOI: 10.1093/cid/ciy874
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















