Ramsay-Hunt syndrome associated with meningitis and vasculitis in an immunocompetent individual
DOI:
https://doi.org/10.5935/2764-734X.e20240340Keywords:
Herpes Zoster Oticus, Central Nervous System, Vasculitis, Meningitis Viral, Varicella Zoster Virus Infection, Case ReportAbstract
The varicella zoster virus (VZV) causes chickenpox as a primary infection and its reactivation leads to herpes zoster, which is a painful vesicular rash with dermatomal distribution associated with neuralgia. It may also be reactivated in the central nervous system in the form of meningitis, meningoradiculitis, myelopathy, vasculitis and Ramsay-Hunt syndrome. In this report, we describe the case of a previously healthy 22-year-old man who presented with right hemiplegia and peripheral facial paralysis associated with painful vesicles in the ipsilateral auditory pavilion, accompanied by fever and headache. Magnetic resonance imaging of the brain, with a study of the arterial vessels, revealed findings compatible with vasculitis in the long segment of the left internal carotid artery. Although molecular biology tests of cerebrospinal fluid for herpes family viruses were negative, high titers of immunoglobulin G antibodies to varicella zoster in two different peripheral blood samples were observed. Two weeks after initiating treatment with parenteral acyclovir, the patient was asymptomatic and his neurological deficits improved significantly. This report highlights the importance of VZV reactivation as a cause of meningitis and vasculitis in young, immunocompetent individuals without comorbidities.
Downloads
INTRODUCTION
Varicella zoster virus (VZV) is a human herpes virus that causes chickenpox as a primary infection1,2. VZV can reactivate and cause herpes zoster and post-herpetic neuralgia as the host’s cellular immunity decreases3. VZV can manifest as meningitis, encephalitis, myelopathy, vasculitis and Ramsay-Hunt syndrome (RHS) when reactivated in the central nervous system (CNS)4,5.
RHS is characterized by the association of optic herpes zoster with peripheral facial paralysis5,6. In VZV vasculitis, latent viruses reactivation takes place in the trigeminal ganglia up to the cerebral arteries1,4,7,8. The most common manifestations are stroke, dissection and venous sinus thrombosis4,6,9,10.
The diagnosis of neurological reactivations is supported by the temporal relationship between the onset of symptoms and the presence of the rash11,12. Laboratory confirmation consists of the detection of VZV genetic material (DNA-VZV) or IgG anti-VZV antibodies in the cerebrospinal fluid7,10,13. Neuroimaging can also help confirm the diagnostic suspicion14. The preferred treatment for neurological manifestations is intravenous acyclovir, which should be started in the acute phase of the disease as it is associated with a reduction in the incidence of strokes4,5,15,16.
In this study, we report on a patient with meningitis and vasculitis caused by VZV, followed by RHS. This is an uncommon presentation, due to both the patient’s characteristics and the site of reactivation, with a favorable outcome after diagnosis and the institution of antiviral treatment.
CASE REPORT
A 22-year-old man with no known comorbidities presented. For teaching purposes, Figure 1 shows the chronological sequence of the main events.
Figure 1. Timeline with the main events that took place during the reported case.
About 15 days ago, the patient sought medical attention in his city of origin (São Carlos, in the state of São Paulo) complaining of loss of muscle strength on the right side. There, he was diagnosed with ischemic stroke and prescribed platelet antiaggregants. However, after a week, he developed a holocranial headache associated with fever, vomiting and painful vesicular lesions in the right ear canal and pinna. He was diagnosed with acute otitis media and prescribed oral antibiotics. As there was no clinical improvement, the patient went to another service where a lumbar puncture was performed for investigation. He remained hospitalized at that service for seven days, before being transferred to an infectious diseases hospital in São Paulo city. The referral form showed a positive result for India ink staining in the cerebrospinal fluid (CSF), and he was prescribed amphotericin B (without further information on dosage) for the treatment of cryptococcal meningitis. He was readmitted now in good general condition, hemodynamically stable and afebrile. The first assessment by the neurology team found peripheral facial paralysis and complete hemiparesis on the right, as well as an exaggerated triceps reflex and plantar cutaneous extension on the right. Magnetic resonance imaging (MRI) of the skull showed a recent ischemic lesion in the left nucleocapsular region, in the territory of the anterior choroidal artery (Figure 2, images A to D). A new lumbar puncture was performed, with CSF analysis revealing lymphomonocytic pleocytosis. There was no evidence of any etiological agent (including negative lateral flow tests for Cryptococcus spp. in CSF and in a blood sample obtained by capillary puncture). These negative results were consistent in subsequent CSF punctures, as were the repeated negative polymerase chain reaction (PCR) tests for herpes viruses shown in Table 1. Serologies for syphilis, hepatitis and HIV were non-reactive. Serum IgG antibodies against VZV, on the other hand, reached titers of more than 4,000 mUI/mL (for a reference value of less than 150 mUI/mL). Given the clinical diagnosis of RHS associated with meningitis, both of which were possibly caused by VZV (although not confirmed in CSF analysis), treatment was started with intravenous acyclovir 750 mg three times a day. It was also decided to continue the treatment for cerebral cryptococcosis with amphotericin B deoxycholate 50 mg/day and fluconazole 200 mg twice a day, until the case could be better clarified. Magnetic resonance angiography (MRA) of the brain was then carried out using a protocol for evaluating the vascular wall (“black blood”), which showed the presence of an inflammatory process in the middle and muscular layers of the long segment of the internal carotid artery (in the intraand extracranial portions) on the left (Figure 3). The differential diagnoses made based on this evidence were vasculitis or dissection of the left internal carotid artery, the latter being less likely. In screening tests for autoimmune diseases, carried out to investigate the differential diagnosis of CNS vasculitis, autoantibody tests were negative. After two weeks of treatment with parenteral acyclovir, the patient was already showing significant improvement in the initial neurological deficits. In view of this, the initial hypothesis of cerebral cryptococcosis was questioned in a multidisciplinary discussion of the case, and it was concluded that there had been a reading error in the India ink test of the first CSF sample, corroborated by its negativity in the other samples. Also, the neuroimaging findings did not suggest this diagnosis. The antifungal medications were therefore discontinued after two weeks of use. The patient completed 20 days of treatment with acyclovir (associated, in the first week, with oral prednisone at a dose of 1 mg/kg/day) after which period he remained hospitalized awaiting, among other things, reversal of the acute kidney injury secondary to the medication. In the sixth week of hospitalization, he was discharged asymptomatic; a new IgG serum antibody test against VZV was taken only for documentation purposes (considering that the serology does not represent a cure criterion) and IgG titers remained above 4,000 mUI/mL in this second sample. On his first outpatient visit, after one month, the patient remained asymptomatic and had no motor or sensory sequelae. His most recent brain MRI (carried out two years after the episode described) shows that there is still an ischemic gap between the knee and the posterior arm of the left internal capsule (Figure 2, images E and F).
| 2nd day of hospitalization | 18th day of hospitalization | 24th day of hospitalization | |
|---|---|---|---|
| Color | Appearance | Colorless | clear | Colorless | clear | Colorless | clear |
| Proteins /mm 3 | 31 | 37 | 24 |
| Glucose /mm 3 | 51 | 49 | 56 |
| Red blood cells /mm 3 | 0 | 2 | 20 |
| Yeasts /mm 3 | 0 | 0 | 0 |
| Lactate (mg/dL) | 12 | 12 | 12 |
| Total cell count /mm 3 | 180 | 22 | 22 |
| % Neutrophils | Macrophages | Lymphocytes | Monocytes | 0 | 6% | 82% | 7% | 0 | 0 | 92% | 6% | 0 | 0 | 90% | 8% |
| Bacterioscopy (GRAM) | Negative | Negative | Negative |
| Aerobic culture | Negative | Negative | Negative |
| Fungal culture | Negative | Negative | Negative |
| Lateral flow test for Cryptococcus spp . | Negative | Negative | Negative |
| India Ink | Negative | Negative | Negative |
| Alcohol-acid resistant bacilli | Negative | Negative | Negative |
| PCR for M. tuberculosis | Not detected | Not detected | Not detected |
| PCR for herpes virus type 1 | Not detected | Not detected | Not detected |
| PCR for herpes virus type 2 | Not detected | Not detected | Not detected |
| PCR for varicella | Not detected | Not detected | Not detected |
| PCR for cytomegalovirus | Not detected | Not detected | Not detected |
| PCR for Epstein-Barr virus | Not detected | Not detected | Not detected |
Figure 2. MRI scan of the skull carried out in the first week of hospitalization. In the first two rows, the blue arrows in images A (FLAIR), B (T2), C (diffusion) and D (T1 post-gadolinium) indicate the recent ischemic lesion in the left nucleocapsular region involving the posterior arm of the internal capsule on this side (anterior choroidal artery territory). The third row shows images E (T2) and F (T1 post-gadolinium) from the patient's follow-up examination, two years after the reported event, showing a chronic lacunar infarction in the left nucleocapsular region.
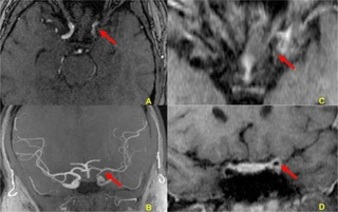
Figure 3. Arterial magnetic resonance angiography (MRA) images of the skull with a study of the vessel wall ("black blood") at the time of the ischemic event. In A, the images show stenosis and irregularities in the contours of the left internal carotid artery in the supraclinoid segment. In B (oblique coronal plane), the image shows a reduction in the flow signal in the left internal carotid artery, especially in its supraclinoid segment. In C (axial plane) and D (coronal plane), the images show parietal thickening with concentric post-gadolinium enhancement in the supraclinoid segment of the left internal carotid artery. The anterior choroidal artery emerges from the posterior portion of this segment. The red arrows indicate the changes described herein.
DISCUSSION
Epidemiological databases estimate a worldwide prevalence of 95% of people already exposed to the VZV3,7,13,15. The present report is a case of dermatological and neurological reactivation of VZV in an uncommon form: RHS and arterial vasculitis5,10. Although factors that interfere with the host’s cellular immunity are decisive in the clinical expression of VZV reactivation, viral virulence factors are also important, as genetic mutations induce strains to express different phenotypes in humans1,3,10,11. It is also worth noting that CNS reactivations can occur even in immunocompetent patients5.
It is important to note the sequence of manifestations presented by our patient: focal neurological symptoms (diagnosed as a stroke) came first, while the herpetic lesions appeared on the skin a week later. Approximately one in three patients with VZV vasculitis has no concomitant dermatological condition9,11,12. In our case, the patient also presented other findings compatible with herpetic meningitis, such as lymphomonocytic pleocytosis in CSF analysis, which, according to the literature, is a common feature in this context1,6,9.
Molecular biology techniques contribute significantly to the definition of the etiological diagnosis. Tests for VZV DNA in CSF are often positive in the first 2 weeks of infection and are undetectable thereafter; therefore, a negative test does not exclude a diagnosis in cases with a longer evolution8. In our case, the non-detection of VZV DNA in the lumbar punctures by the PCR technique (Table 1) can be explained by the days of symptoms that the patient had already experienced (Figure 1). The identification of IgG anti-VZV antibodies in CSF, on the other hand, has relevant and superior diagnostic value when compared to molecular diagnosis, particularly in cases of vasculitis, when the onset of symptoms occurs at around four weeks after reactivation of the virus1,5. In the present case, IgG anti-VZV antibodies could not be tested in CSF due to its unavailability in the service. However, high IgG anti-VZV antibody titers were detected in two different peripheral blood samples (in the first and sixth weeks of hospitalization), which was of great value in defining diagnosis. Most case reports in the literature do not refer to the use of this diagnostic tool in the blood, since it has been already used in CSF. However, the detection of IgG anti-VZV antibodies in peripheral blood may be another possible laboratory evidence (in our case, the only one) of the presence of VZV.6,10
As far as radiological aspects are concerned, imaging tests are of fundamental importance because they help to monitor progress through comparative analyses and differentiate cases of VZV vasculitis from those of other etiology. Neuroimaging in cases of VZV vasculitis shows superficial and deep lesions, especially at the junction of white and gray matter1,6,7,16. These changes are due to pathological remodeling of the cerebral arteries caused by reactivation of the latent virus in the cranial nerve ganglia, followed by transaxonal dissemination to the adventitial layer of the cerebral arteries2,4,7,16. Cerebral angiography can show constrictions in the vessels, followed by dilation downstream1,14,16. MRA with the “black blood” protocol, as demonstrated in this case, shows vessel thickening and enhancement, which are proportionally related to the degree of inflammation of CNS vasculitis1,2,8,10.
The importance of adequate treatment has been supported by retrospective cohort studies and systematic reviews, which have shown that the risk of ischemic stroke is doubled in the first 30 days after VZV reactivation4,16, and is even higher in patients who did not receive antiviral treatment during the acute phase15. In the present case, clinical reasoning was hampered by the previous misdiagnosis of neurocryptococcosis; however, this did not prevent the initiation of therapy aimed at VZV, which certainly led to better outcomes. The treatment of choice for cases of VZV reactivation in the CNS is intravenous acyclovir at a dose of 10-15 mg/kg/day, three times a day.2,3,5. Some patients benefit from oral valacyclovir for a few more months after stopping acyclovir, its discontinuation being guided by the resolution of symptoms8. The adjuvant role of oral prednisone at a dose of 1 mg/kg/day at the start of treatment is due to the large number of inflammatory cells in the arteries infected by the virus; however, prolonging its use is not recommended because it potentiates viral replication 2,13,16.
This patient’s history of chickenpox in childhood and his vaccination records were not known. Currently available vaccines against herpes zoster include Zostavax® (a live attenuated vaccine with a single dose) and Shingrix® (inactivated vaccine with two doses at intervals of two to six months). Their main role is to prevent new episodes of herpes zoster and post-herpetic neuralgia17. In Brazil, Shingrix® is recommended for healthy individuals over 50 years of age (regardless of whether they have had episodes of zoster or have already received the attenuated vaccine) and can also be administered to individuals over 18 years of age who are immunodepressed17. However, to date, no study has demonstrated the benefits of immunization in relation to reactivations of the virus in the CNS2,3,13. In fact, the patient in our case should not have received the inactivated vaccine because he was immunocompetent and he was below the minimum recommended age. Shingrix® has not yet been included in the Brazilian Unified Health System (SUS) National Immunization Program and is currently available only in private vaccination clinics.
CONCLUSION
This report highlights the importance of including VZV reactivation as a differential diagnosis for meningitis and vasculitis in young, immunocompetent individuals. In the present case, RHS following the neurological condition made it possible to attribute the clinical manifestations to the same etiology agent. However, the spectrum of manifestations of VZV reactivation is broad, and all available laboratory and neuroimaging tools should be used to establish an early diagnosis and to guide appropriate treatment.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731-40. DOI: 10.1016/S1474-4422(09)70134-6
2. Marra CM. Infectious and postinfectious vasculopathies. Neuroimaging Clin N Am. 2024;34(1):13-21. DOI: 10.1016/j.nic.2023.06.001
3. Maher MD, Douglas VP, Douglas KAA, Collens SI, Gilbert AL, Torun N, et al. Clinical and neuroradiologic characteristics in varicella zoster virus reactivation with central nervous system involvement. J Neurol Sci. 2022;437:120262. DOI: 10.1016/j.jns.2022.120262
4. Gilden D, Nagel M, Cohrs R, Mahalingam R, Baird N. Varicella zoster virus in the nervous system. F1000Res. 2015;4(Faculty Rev-1356):1356. DOI: 10.12688/f1000research.7153.1
5. Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system – Prognosis, diagnostics and treatment. J Infect. 2015;71(3):281-93. DOI: 10.1016/j.jinf.2015.06.004
6. Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27(3):356-60. DOI: 10.1097/WCO.0000000000000092
7. Bakradze E, Kirchoff KF, Antoniello D, Springer MV, Mabie PC, Esenwa CC, et al. Varicella zoster virus vasculitis and adult cerebrovascular disease. Neurohospitalist. 2019;9(4):203-208. DOI: 10.1177/1941874419845732
8. Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, Russman A et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77(4):364-70. DOI: 10.1212/WNL.0b013e3182267bfa
9. Gilden D. Varicella-zoster virus infections. Continuum (Minneap Minn). 2015;21(6):1692-703. DOI: 10.1212/CON.0000000000000246
10. Carod Artal FJ. Clinical management of infectious cerebral vasculitides. Expert Rev Neurother. 2016;16(2):205-21. DOI: 10.1586/14737175.2015.1134321
11. Kennedy PG, Mogensen TH. Determinants of neurological syndromes caused by varicella zoster virus (VZV). J Neurovirol. 2020;26(4):482-95. DOI: 10.1007/s13365-020-00857-w
12. Chiang F, Panyaping T, Tedesqui G, Sossa D, Costa Leite C, Castillo M. Varicella zoster CNS vascular complications. A report of four cases and literature review. Neuroradiol J. 2014;27(3):327-33. DOI: 10.15274/NRJ-2014-10037
13. Nagel MA, Niemeyer CS, Bubak AN. Central nervous system infections produced by varicella zoster virus. Curr Opin Infect Dis. 2020;33(3):273-8. DOI: 10.1097/QCO.0000000000000647
14. Cheng-Ching E, Jones S, Hui FK, Man S, Gilden D, Bhimraj A, et al. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci. 2015;351(1-2):168-73. DOI: 10.1016/j.jns.2015.02.017
15. Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58(11):1497-503. DOI: 10.1093/cid/ciu098
16. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16(2):12. DOI: 10.1007/s11910-015-0614-5
17. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde e Ambiente. Departamento de Imunizações e Doenças Imunopreveníveis. Manual dos Centros de Referência para Imunobiológicos Especiais [Internet]. 6ª ed. Brasília: Ministério da Saúde; 2023; [acesso em 31 de janeiro de 2024]. Disponível em: https://sbim.org.br/images/calendarios/manual-dos-centros-de-referencia-para-imunobiologicos-especiais-6a-edicao-2023.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















