Tuberculous pyomyositis
DOI:
https://doi.org/10.5935/2764-734X.e20240442Keywords:
Tuberculosis Extrapulmonary;, Pyomyositis, Abscess, Diabetes Mellitus, Case report.Abstract
Musculoskeletal tuberculosis is a rare form of extrapulmonary manifestation of the disease, with few reports in the medical literature. This makes it difficult to diagnose and manage promptly. We report the case of a patient with tuberculous pyomyositis in multiple muscle groups whose only previous pathology was controlled type II diabetes mellitus. The diagnosis was established by performing a rapid molecular test (GeneXpert Mtbc/RIF®) on purulent material collected by aspiration puncture of the dorsal vertebral body. The patient underwent drug treatment for tuberculosis with a regimen of rifampicin, isoniazid, pyrazinamide, and ethambutol (RHZE) for 2 months in an intensive phase, followed by maintenance with rifampicin and isoniazid for another 10 months, with complete resolution of the collections.
Downloads
INTRODUCTION
Musculoskeletal tuberculosis is a rare form of extrapulmonary manifestation of the disease, accounting for 3% of diagnosed cases. It mainly affects the bones and joints, with the main clinical forms being spondylodiscitis, osteomyelitis, and tuberculous arthritis1. Specific involvement of the skeletal muscles is an even rarer event, with a prevalence of around 1%2. Few reports in the medical literature about this clinical entity give it a certain “oblivion” as a diagnostic hypothesis compared to other inflammatory, infectious, and even neoplastic diseases with similar clinical manifestations and radiological images. This makes it difficult to recognize and facilitates a timely diagnosis and the start of appropriate treatment3. This report aims to describe the case of a 58-year-old male patient with a history of controlled type II diabetes mellitus and multiple abscesses concomitantly affecting several muscle groups.
CASE REPORT
A 58-year-old male patient, born in Bahia but living in São Paulo for over 30 years, came to the emergency room due to nodular, painful, hyperemic lesions with fluctuation points and spontaneous drainage in both armpits, as well as presenting an area of hyperemia with a central drainage point in the sternum. This condition started 4 months ago when the patient was hospitalized for 14 days in another service, where he received intravenous antibiotic therapy (he could not name the medication). He was discharged from the hospital with a prescription of cephalexin for 7 days more, but his condition did not improve. The following month, new lesions with a similar appearance appeared on the left wrist and bilateral inguinal region, and the patient also noticed bulges in the left dorsal and gluteal regions, which were painless and showed no phlogistic signs. The most recent outflow of pus from the sternal region was the main reason for this visit. The patient reported no fever, sweating, weight loss, respiratory symptoms, or other associated signs. He was taking metformin 500 mg twice a day to treat type II diabetes mellitus and reported good glycemic control.
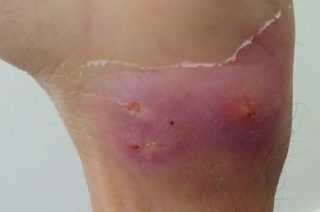
On admission, he was in good overall health with a body mass index of 24 kg/m2, weighing 65 kg, and hemodynamically stable. Physical examination revealed hyperemia in the sternal region with a spontaneous drainage hole (Figure 1) and hyperemic nodules in the axilla, which had no drainage point or fluctuation. There was also coalescent lymph node enlargement, hardened and adhered to the deep planes in the bilateral inguinal region, a floating bulge (but without phlogistic signs) in the dorsal region, as well as hyperemic nodular lesions with drainage points in the left wrist (Figure 2).
Figure 1. Hyperemic lesion in the sternal region with central drainage hole.
Figure 2. Hyperemic nodular lesions on the left wrist with multiple drainage points.
Initial laboratory tests revealed hemoglobin of 9.4 g/dL (reference value: 13.5-17.5 g/dL), hematocrit of 28% (reference value: 40%-52%), total leukocytes of 6,100 cells/mm³ (reference value: 4.0-11.0 thousand/ mm3), with 4,900 neutrophils (reference value: 2.50-7.50 thousand/mm³) and 700 lymphocytes (reference value: 1.50-3.50 thousand/mm3), as well as 299 thousand platelets per mm³ (reference value: 150-450 thousand/mm³). Urea, creatinine, and glucose levels were 40 mg/dL (reference value: 19-43 mg/dL), 0.7 mg/dL (reference value: 0.66-1.25 mg/dL), and 156 mg/dL (reference value: 70-99 mg/dL), respectively. C-reactive protein was high at 62.9 mg/L (reference value: <5 mg/L). Serology for HIV I and II, syphilis, and hepatitis B and C were non-reactive.
A chest computed tomography (CT) scan revealed liquid collections on the anterior chest wall, adjacent to the sternum, the largest measuring 10.0 × 9.8 × 1.0 cm3 on the right, with peripheral contrast enhancement (Figure 3). Significant axillary lymph node enlargement with a necrotic center was also observed, while there were no alterations in the lung parenchyma. CT scans of the abdomen and pelvis showed collections in the ileopsoas, pre- and retro-sacral muscles, and gluteal muscles, the largest on the left, located in the myoadipose planes of the pelvic cavity on the right and in the bilateral inguinal region, measuring 9.5 cm, with peripheral enhancement (Figures 4 5). There was also bilateral inguinal lymph node enlargement of up to 2.9 cm.
Figure 3. An axial computed tomography scan showing extrapleural collections near the anterior portion of the upper third of the chest bilaterally, with peripheral contrast enhancement and extension to the pectoral muscles. There is also bilateral axillary lymph node enlargement with areas of central necrosis.
Figure 4. Computed tomography scan in the coronal reconstruction of the abdomen, showing fluid collections in the ileopsoas muscle bilaterally.
Figure 5. Computed tomography scan in the coronal reconstruction of the abdomen showing coalescent liquid collections affecting the bilateral gluteal muscles, more so on the left. There is another collection in the presacral region.
During the investigation, peripheral blood cultures were taken for aerobic and anaerobic bacteria, fungi, and mycobacteria, which were negative, with no growth of microorganisms. Direct aspiration puncture of the collection in the dorsal region resulted in the sampling of 10 ml of purulent content. On the second day of hospitalization, ceftriaxone (1 g intravenously every 12 hours) combined with clindamycin (600 mg intravenously every 8 hours) was started empirically due to the main diagnostic hypothesis of tropical pyomyositis. The following day, however, the molecular test for Mycobacterium tuberculosis (GeneXpert Mtbc/ RIF®) carried out on the previously collected pus was positive, with sensitivity to rifampicin. All the other microbiological tests on that same lumbar sample were negative. On the fourth day of hospitalization, a regimen of four tablets in a single conjugated dose of rifampicin, isoniazid, pyrazinamide, and ethambutol (RHZE) was started, and the prescription for ceftriaxone was suspended.
To assess possible bone involvement in the vertebral bodies, the patient underwent magnetic resonance imaging of the thoracic and lumbosacral spine, which showed multiple collections with peripheral impregnation by the contrast medium in the perivertebral region of the lumbosacral spine and, at the thoracic level, at T8-T9. There was also a collection located in the subcutaneous tissue of the posterior sacral paravertebral region (measuring 15.6 × 11.0 × 4.2 cm3) and another in the myoadipose planes of the left gluteal region. There was a third collection in the pelvic cavity, notably in the presacral space, which extended from L4 to S3 (Figures 5 6). However, no signs of bone involvement were identified.
Figure 6. An axial computed tomography scan of the pelvis showing liquid collections with peripheral enhancement in the subcutaneous tissue of the lumbar region bilaterally, more so on the right, as well as bilateral iliac lymph node enlargement with central necrosis and collections in the ileopsoas muscle on the right.
The patient was discharged on the fifth day of hospitalization for follow-up in an outpatient clinic specializing in tuberculosis. No surgical approach was performed during the entire course of the case. The four-tablet RHZE regimen lasted for 2 months (the intensive phase of tuberculosis treatment), at the end of which there was complete remission of the lesions on the wrist, sternum, inguinal and axillary regions, as well as all the deep liquid collections, with the due tomographic documentation. All the samples collected and seeded on a culture medium for mycobacteria showed no growth after 42 days of incubation. However, it was decided to extend the "maintenance period" of treatment with rifampicin and isoniazid for 10 months more, making a total of 12 months of specific tuberculosis treatment.
DISCUSSION
Tuberculosis can spread to muscle tissue through three main mechanisms. In 29% of cases, it spreads hematogenously from the primary pulmonary focus. Most cases (63%) are caused by the involvement of nearby tissues due to adjacency. In 8% of diagnoses, the pathogen is directly inoculated4. In a Spanish study including 2224 patients diagnosed with tuberculosis, only four cases had pyomyositis5. One possible explanation for the rarity of this extrapulmonary form is that skeletal muscle tissue is an environment with low oxygen tension and a high concentration of lactic acid, and there is a shortage of endothelial reticulum tissue cells around it, creating an inhospitable environment for mycobacteria to survive and multiply4,6.
Despite its rarity, it is presumed that there are more cases of tuberculous pyomyositis in countries with high endemicity. In a retrospective study carried out in Taiwan, 35 out of 1153 patients with a microbiological or histological diagnosis of tuberculosis were diagnosed with pyomyositis7. Of these, only 29% had some underlying immunosuppressive comorbidity, and 25% had diabetes mellitus, as in the case described in our report. Another interesting finding of the same study was the presence of lung lesions concomitant with pyomyositis in 51% of cases, allowing microbiological diagnosis in the sputum in 44.5% of these patients7. This was not the case with our patient, who had no respiratory manifestations and normal parenchyma on the CT scan. Even so, investigation of pulmonary involvement is mandatory, and sputum collection can be an important diagnostic tool in patients with any suspicion of extrapulmonary tuberculosis.
The diagnosis of tuberculous pyomyositis depends on high clinical suspicion, both because of its non-specific presentation and because doctors are unfamiliar with this form of the disease6. Its main differential diagnosis is tropical pyomyositis, whose etiologic agent, in most cases, is the gram-positive bacterium Staphylococcus aureus8 The evolution of tuberculous pyomyositis tends to be more indolent than tropical pyomyositis, with more non-specific symptoms such as fever, weight loss, and sweating and less pain in the affected muscle group9. Other less common differential diagnoses in this context are neoplasms affecting the skeletal muscles, hematomas, deep vein thrombosis, and muscle abscesses caused by other pathogens such as fungi and, more specifically, in the case of the ileopsoas muscle, complications of appendicitis and diverticulitis6.
With regard to imaging tests, magnetic resonance imaging stands out as the best method for assessing soft tissues10, although it does not allow differentiation between the various etiologies. In a radiological study evaluating 136 patients with muscle abscesses, the predictors of bacterial pyomyositis were a history of diabetes mellitus, extra-spinal involvement, and hyper-intense enhancement of the abscess wall on T211.
The histopathological finding of caseous granulomas in muscle tissue biopsies is highly suggestive of tuberculous pyomyositis, but growth in culture of the purulent samples is the gold standard described in the literature (a test that was, however, negative in our patient)6. Another important diagnostic tool is detecting genetic material from the Mycobacterium tuberculosis complex using the polymerase chain reaction (PCR). In a study that included different samples from 182 patients with suspected extrapulmonary tuberculosis, PCR showed the highest performance compared to the other techniques (bacilloscopy and culture) in the analysis of pus.12 This was, in fact, the case with our patient, whose bacilloscopy and culture of the punctured fluid had negative results.
As for treatment, 82.9% of the patients in Taiwan underwent some kind of surgical approach to drain the abscesses (31% of them through more than one procedure), and this was also the approach adopted in most of the other reports in the literature3,4,5,6,7. However, the relative lack of painful symptoms and the rapid clinical response to the RHZE regimen in our case justified opting for exclusively drug treatment. Another difference with our patient (always in good general condition and with no signs of sepsis) was his good evolution compared to the high lethality rate (14.3%) of the other cases, reaching 30% in patients with pyomyositis due to hematogenous dissemination (as in our case). These patients usually die as a result of a septic condition with no other microorganisms identified, and patients taking glucocorticoids tend to have a worse outcome7.
Whether or not surgical drainage is chosen is not a reason to standardize or adopt this approach as a service routine, not least because this approach is sometimes unavoidable in relieving symptoms or even collecting representative samples. The fact is that when extrapulmonary tuberculosis is suspected, clinical experience has avoided, whenever possible, punctures and the placement of drains that could perpetuate purulent or lymphatic fistulas13. Conversely, the downside of not surgically draining tuberculous abscesses may be an extended treatment maintenance period; in our case, the isoniazid + rifampicin regimen was maintained for 10 months. Although there is no formal recommendation in the literature regarding this course of action, the reasoning was based on the size of the collections and the number of muscle groups affected. In any case, any conservative treatment (such as the one reported here) implies the guarantee of close and frequent monitoring of the patient for a prolonged period of time, even on an outpatient basis, until the criteria for curing the infection are effectively met.
CONCLUSION
There are few reports in the medical literature on tuberculous pyomyositis, and the case presented here stands out for having overcome the usual diagnostic difficulty and the consequent delay in introducing treatment by immediately including this suspicion in the repertoire of diagnostic possibilities attributed to the patient. Once the suspicion has been raised, it is up to the medical team to order the relevant, specific, and essential microbiological tests in the context of any infection caused by mycobacteria, especially molecular biology techniques, which have brought speed and high sensitivity to the investigative process.
"This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited."
References
1. Al-Khazraji A, Takher J, Alkhawam H, Fabbri M. Primary Tuberculous Pyomyositis of the Calf Muscles. Am J Med Sci. 2017;353(2):187-8. DOI: 10.1016/j.amjms.2016.05.010
2. Narayanappa G, Nandeesh BN. Infective myositis. Brain Pathol. 2021;31(3):e12950. DOI: 10.1111/bpa.12950
3. Puttick MP, Stein HB, Chan RM, Elwood RK, How AR, Reid GD. Soft tissue tuberculosis: a series of 11 cases. J Rheumatol [Internet]. 1995; [cited 20 Feb 2024]; 22(7):1321-5. Available from: https://pubmed.ncbi.nlm.nih.gov/7562766/
4. Murugesh AS, Edwin FM, Srinivasaprasad ND, Sujit S, Thirumalvalavan K. Tuberculous myositis and cellulitis in a renal transplant recipient. Indian J Tuberc. 2020;67(3):353-6. DOI: 10.1016/j.ijtb.2019.04.010
5. Rafaela S, Esther R, Carmen R, Marta S. MRI of musculoskeletal extraspinal tuberculosis. J Comput Assist Tomogr. 2001;25(2):177-83. DOI: 10.1097/00004728-200103000-00004
6. Simopoulou T, Varna A, Dailiana Z, Katsiari C, Alexiou I, Basdekis G, et al. Tuberculous pyomyositis: a re-emerging entity of many faces. Clin Rheumatol. 2016;35(4):1105-10. DOI: 10.1007/s10067-014-2564-8
7. Wang JY, Lee LN, Hsueh PR, Shih JY, Chang YL, Yang PC, et al. Tuberculous myositis: a rare but existing clinical entity. Rheumatology (Oxford). 2003;42(7):836-40. DOI: 10.1093/rheumatology/keg228
8. Habeych ME, Trinh T, Crum-Cianflone NF. Purulent infectious myositis (formerly tropical pyomyositis). J Neurol Sci. 2020:413:116767. DOI: 10.1016/j.jns.2020.116767
9. Narang S. Tuberculous pyomyositis of forearm muscles. Hand (NY). 2009;4(1):88-91. DOI: 10.1007/s11552-008-9127-x
10. Abdelwahab IF, Bianchi S, Martinoli C, Klein M, Hermann G. Atypical extraspinal musculoskeletal tuberculosis in immunocompetent patients: part II, tuberculous myositis, tuberculous bursitis, and tuberculous tenosynovites. Can Assoc Radiol J [Internet]. 2006; [cited 20 Feb 2024]; 57(5):278-86. Available from: https://pubmed.ncbi.nlm.nih.gov/17265982/
11. Thammaroj P, Panitchote A, Muktabhant C, Chowchuen P. Discrimination between tuberculous and bacterial pyomyositis in magnetic resonance features. Eur J Radiol Open. 2020:7:100214. DOI: 10.1016/j.ejro.2020.01.003
12. Ajantha GS, Shetty PC, Kulkarni RD, Biradar U. PCR as a diagnostic tool for extra-pulmonary tuberculosis. J Clin Diagn Res. 2013;7(6):1012-5. DOI: 10.7860/JCDR/2013/5425.3075
13. Lai YF, Chao TY, Wang YH, Lin AS. Pigtail drainage in the treatment of tuberculous pleural effusions: a randomised study. Thorax. 2003;58(2):149-51. DOI: 10.1136/thorax.58.2.149
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















