Herpes zoster and polyarteritis nodosa: diagnostic challenges
DOI:
https://doi.org/10.5935/2764-734X.e202112004Keywords:
Polyarteritis nodosa, Systemic vasculitis, Algorithms, Varicella-zoster virus infection, Case ReportAbstract
A 74-year-old female patient came to the public university hospital due to pain in her lower limbs for 2 decades, associated with a trophic lesion on the lateral side of her right leg for 3 months and consumptive syndrome for 2 months. The patient treated herpes zoster virus for 4 years in another medical service. After anamnesis, physical examination and the use of a specific primary vasculitis algorithm, the patient was diagnosed with polyarteritis nodosa, allowing the initiation of appropriate therapy to improve the clinical condition. Morphologically, the histological study evidenced transmural necrotizing inflammation of medium and small caliber arteries. The reported clinical case reflects the need to conduct a more efficient etiological investigation in patients with primary vasculitis.
Downloads
INTRODUCTION
The varicella-zoster virus (human herpesvirus 3) is a neurotropic DNA virus with sensory roots that mainly affects individuals immunocompromised by specific drugs or autoimmune diseases.1,2,3
In the acute phase, the disease is characterized by neuritis associated with skin eruptions that serve as transmission sites until they lose their vesicular component in the chronic phase, when the patient may present with post-herpetic neuralgia.1,4
However, in cases of retinal necrosis or Ramsay Hunt syndrome, herpes zoster is usually treated empirically, based on clinical diagnosis rather than precise diagnostic confirmation (for example, by polymerase chain reaction analysis). As a result, other diseases may be overlooked.2, 5, 6
This inadvertent viral therapy in patients with polyarteritis nodosa (PAN) remains unreported in the literature among the neuropathic diseases with a differential diagnosis for human herpesvirus 3.
CASE DESCRIPTION
A 74-year-old female patient with lower limb pain for 24 years and an infected trophic lesion on the lateral aspect of the right leg for 3 months was referred to the Municipal University Hospital.
She reported being bedridden for four years due to herpes zoster neuropathy and receiving acyclovir treatment at another hospital. There was no record that she received herpes vaccine. Due to the painful condition, the patient had been medicated with a morphine pump, chlorpromazine, and amitriptyline. She had lost 8 kg in the last two months.
The patient irregularly took losartan and atenolol for high blood pressure.
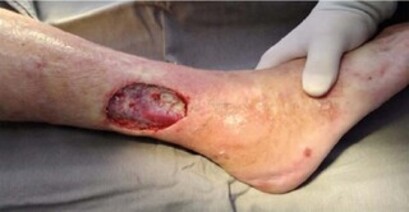
She was lucid and coherent on physical examination, hemodynamically stable, and hypertensive (160x96 mmHg blood pressure; 98 bpm heart rate). The lower limbs revealed thermal gradient changes, increased capillary refill time, livedo reticularis, peripheral cyanosis, and an infected trophic lesion measuring approximately 7.0x5.0 cm on the lateral compartment of the right leg. Despite motor and sensory deficits in both lower limbs, pulses were present and symmetrically distributed.
Due to the wasting syndrome associated with neuropathy and altered lower limb perfusion, the Bezerra et al. algorithm was used, which suggested polyarteritis nodosa (PAN) over Varicella zoster (Figure 1).
Figure 1. Algorithm developed by Bezerra et al. (11)
Considering this new hypothesis, a biopsy was performed during the skin lesion debridement to confirm the diagnosis (Figure 2). The pathological examination discovered a transmural leukocyte infiltrate (Figure 3) as well as fibrinoid necrosis with polymorphonuclear infiltrate (Figure 4).
Figure 2. Post-surgical debridement of an infected ulcer in the right leg with devitalized tissue.
In turn, varicella-zoster has been addressed in several literature reviews, with the unspecified signs and symptoms as a factor of diagnostic error. Facial symptoms of other diseases, such as stroke, frequently mimic herpes infection of the trigeminal nerve. The same thing happens when the virus infiltrates thoracic and lumbar dermatomes, which are known to be virus-favored anatomical regions. In all these conditions, pain associated with sensory and motor neuropathies should be considered and reviewed in their clinical context. 2,3
Figure 3. Skin fragment of the right leg with transmural leukocyte infiltrate (hematoxylin and eosin (HE) staining with 40x magnification)
Figure 4. Fibrinoid necrosis and polymorphonuclear infiltrate in skin biopsy (40x magnification hematoxylin and eosin (HE) staining)
Due to the infection, 1g of ceftriaxone every 12h and 600 mg of clindamycin every 6h were administered. Vasculitis was treated with 20 mg of prednisone every 8h, combined with 300 mg of gabapentin every 12h.
After two months of treatment, the patient was discharged from the hospital. After another three months of outpatient follow-up, the patient was asymptomatic, with complete healing of the lesion, and was able to walk with a walker. Prednisone was reduced to 20 mg and gabapentin was increased to 300 mg daily.
DISCUSSION
PAN is a primary necrotizing mid-vessel vasculitis, with systemic involvement and no anti-neutrophil azurophil granules antibodies (p ANCA, c ANCA).7,8,9
Although some older articles mention leukemia and hepatitis as potential causes, PAN is classified as an idiopathic disease. It has different pathophysiology than necrotizing vasculopathies caused by Hepatitis B virus infection.10
PAN, like other forms of primary vasculitis, is a rare condition with a difficult diagnosis in the clinical practice in most diverse hospitals.9,11
Despite not aiming to establish diagnostic criteria in vasculitis, the Chapel Hill Consensus Conference (CHCC) defined PAN as necrotizing inflammation of medium and small-caliber arteries without glomerulonephritis or involvement of arterioles, capillaries, or venules.7,12
In 1990, the first CHCC found that patients who met three or more of the 10 criteria in Table 1 had 82% sensitivity and 87% specificity for PAN.12,13
| MAJOR WEIGHT LOSS 4Kg |
|---|
| Testicular pain |
| MYALGIA |
| MONONEUROPATHY / POLYNEUROPATHY |
| Elevated serum urea (>40mg/dL or 14.3mmol/L) or creatinine (>1.5mg/dL or 132mmol/L) |
| Hepatitis B infection - old concept |
| Arteriographic changes unrelated to non-inflammatory disease |
| BIOPSY OF SMALL AND MEDIUM CALIBER ARTERIES WITH POLYMORPHONUCLEATED CELLS |
| DIASTOLIC PRESSURE ABOVE 90mm/Hg |
| LIVEDO RETICULAR |
Although the second CHCC, in 2013, simplified the diagnostic parameters of PAN, this case report contains at least six of the possible criteria.7,14
The kidney is commonly the most affected organ in PAN, due to glomerular ischemia without necrosis inflammation. These patients may have mild proteinuria and hematuria, without casts on the urine test.15
However, neuropathy affects more than 70% of PAN patients. Motor and sensory deficits are usually the first clinical manifestation. As a result, diseases like leprosy and Lyme disease become differential diagnoses in patients with multiple mononeuropathies or polyradiculopathies.8,16
Idiopathic skin nodules, which are unrelated to erythema nodosum, are also characteristic of PAN, (characterized by hypersensitivity reaction and septal panniculitis after an infection such as streptococcal pharyngitis, for example).8,11
Although the diagnosis is clinical, the histological characteristics of an affected organ should be confirmed whenever possible, considering the aggressiveness and potential complications of the treatment. Skin shave biopsy is innocuous compared to the morbidity of visceral biopsies.9,17
PAN is characterized histologically by transmural segmental inflammation of muscular arteries and cellular infiltrate with mononuclear cells and polymorphonuclear leukocytes. While arterial wall necrosis is defined as homogeneous eosinophilia, which is referred to as fibrinoid necrosis. Internal and external elastic layers injury is common, causing dilations and aneurysms, with fragments of neutrophil nuclei (leukocytoclasis) in some cases. Unlike other types of primary vasculitis, PAN patients do not present with granulomatous inflammation.7,8,11,17
In this case report, the patient sufered from limb pain for over 20 years. Although often neglected, myalgia is a common symptom of systemic vasculitis. Ushiyama et al. published a study involving 93 patients with vasculitis, all of whom complained of myalgia.18,19
The Bezerra et al. algorithm is a clinical reasoning tool that is particularly useful when treating patients with wasting syndrome caused by vascular injury. 11
In contrast to the treatment of human herpesvirus 3, which may include acyclovir based solely on medical history and physical examination, histological analysis in PAN is required not only for acquiring information for future use (patient follow-up) but also for diagnostic confirmation because some treatments are extremely morbid.1,3,14
Morphologically, the main feature of PAN is transmural necrotic inflammation of mid-vessel arteries, as shown in Figure 3.8,17
The 2C recommendation for PAN treatment is 1mg/kg/day prednisone, with or without other drugs, such as cyclophosphamide in refractory cases with poor prognosis.14,16,20
Although the medical community has not reached a consensus on the best treatment for neuropathy, we use gabapentin as the first-line treatment, particularly in patients with thin fiber neuropathies that are undetectable by electroneuromyography.6
CONCLUSION
This case report emphasizes the importance of efficient etiological research in patients with pain associated with refractory neuropathy, a common combination in an infectious diseases department. High-incidence diseases like zoster can and should be considered, albeit without neglecting a multidisciplinary approach when searching for other diagnostic hypotheses such as PAN.1,2,3,8,11
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Gnann Junior JW, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002 Aug;347(5):340-6.
2. Rosamilia LL. Herpes zoster presentation, management, and prevention: a modern case-based review. Am J Clin Dermatol. 2020 Feb;21(1):97-107.
3. Ehrenstein B. Diagnosis, treatment and prophylaxis of herpes zoster. Z Rheumatol. 2020 Dec;79(10):1009-17.
4. Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA. 2015 Nov;314(20):2172-81.
5. Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007 Apr;114(4):756-62.
6. Hanewinckel R, Drenthen J, Van Oijen M, Hofman A, Van Doorn PA, Ikram MA. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology. 2016 Nov;87(18):1892-8.
7. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013 Jan;65(1):1-11.
8. Criado PR, Marques GF, Morita TC, Carvalho JF. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016 Jun;15(6):558-63.
9. Bezerra AS, Polimanti AC, Oliveira RA, Fürst RVC, Criado PR, Corrêa JA. Early diagnosis and treatment of leukocytoclastic vasculitis: case report. J Vasc Bras. 2020 Jan;19:e20180072.
10. Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994 Feb;37(2): 187-92.
11. Ozen S. The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat Rev Rheumatol. 2017 Jun;13(6):381-6.
12. Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum. 2010 Feb;62(2):616-26.
13. Puéchal X, Pagnoux C, Baron G, Quémeneur T, Néel A, Agard C, et al. Adding azathioprine to remission-induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors: a randomized, controlled trial. Arthritis Rheumatol. 2017 Nov;69(11):2175-86.
14. Bezerra AS, Polimanti AC, Fürst RVC, Corrêa JA. Algorithm for diagnosis of primary vasculitides. J Vasc Bras. 2019 Mar;18:e20180092.
15. Azanza JC, Sarmiento PC, Lia NL, Alexander AS, Modi V. Leukocytoclastic vasculitis: an early skin biopsy makes a difference. Cureus. 2020 May;12(5):e7912.
16. Lightfoot Junior RW, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990 Aug;33(8):1088-93.
17. Ribi C, Cohen P, Pagnoux C, Mahr A, Arène JP, Puéchal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis without poor-prognosis factors: a prospective randomized study of one hundred twenty-four patients. Arthritis Rheum. 2010 Apr;62(4):1186-97.
18. Ushiyama S, Shimojima Y, Ueno KI, Kishida D, Miyazaki D, Sekijima Y. Clinical characteristics of patients with myalgia as the initial manifestation of small and medium-sized vasculitis: a retrospective study. Rheumatol Int. 2020;40:1667-74.
19. Martins-Martinho J, Dourado E, Khmelinskii N, Espinosa P, Ponte C. Localized forms of vasculitis. Curr Rheumatol Rep. 2021 Jul;23(7):49.
20. Krusche M, Ruffer N, Kötter I. Tocilizumab treatment in refractory polyarteritis nodosa: a case report and review of the literature. Rheumatol Int. 2019 Feb;39(2):337-44.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















