Disseminated paracoccidioidomycosis with meningitis without pulmonary involvement
DOI:
https://doi.org/10.5935/2764-734X.e20250249Keywords:
Central Nervous System, Toxoplasmosis Cerebral;, Paracoccidioidomycosis, Acquired Immunodeficiency Syndrome, Case ReportAbstract
Paracoccidioidomycosis is the most common deep mycosis in Latin America, but it is uncommon in people living with HIV/AIDS, particularly neuroparacoccidioidomycosis. This study reports the case of a 33-year-old man, infected with HIV and with severe immunosuppression. The patient presented the “mixed form” of paracoccidioidomycosis, defined by the concomitant presence of acute/subacute and chronic forms, however without pulmonary involvement. The main differential in this context was cerebral toxoplasmosis, with the final diagnosis being obtained by histological analysis of the lymph node and oral mucosa, as well as through an immunological reaction in the cerebrospinal fluid.
Downloads
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic fungal disease endemic to Latin America, and its etiologic agent is the fungus Paracoccidioides spp. The current prevalence of this disease in Brazil is unknown because it has been excluded from the list of notifiable diseases. There are two pathogenic species: P. brasiliensis and P. lutzii1. Genomic sequence analysis of P. brasiliensis complex has identified five distinct lineages (S1a, S1b, PS2, PS3, and PS4). The P. brasiliensis (sensu stricto) strains (S1a and S1b) are commonly found in the southeast and south of Brazil, as well as in other South American countries. The P. americana strain (PS2) is found in Brazil and Venezuela; the P. restrepiensis strain (PS3) occurs in Colombia and less frequently in other South American countries; and the P. venezuelensis strain (PS4) occurs in Venezuela. P. lutzii is distributed more widely in central-west Brazil1.
Clinically, PCM is classified into the following forms: (i) the acute/subacute form, which accounts for 1%-20% of cases and primarily affects children, adolescents, and young adults, with equal distribution among men and women; and (ii) the chronic form, which affects individuals between 30 and 60 years of age and accounts for 80% of cases, with a male-to-female ratio of 15:12. The acute form primarily affects the lymph nodes, liver, and spleen, with invasion of the bone marrow, joint spaces, and Peyer’s patches. The chronic form affects the lungs, mucous membranes (oral, nasal, laryngeal, and tracheal), adrenal glands, lymph nodes, digestive tract, genitourinary system, bones, eyes, and central nervous system (CNS)2.
Neuroparacoccidioidomycosis (NPCM) occurs when the CNS is involved and can result from the reactivation of a primary focus with hematogenous or lymphatic dissemination, or less commonly, reinfection3. The association between PCM and HIV infection is less common than that of other systemic mycoses (histoplasmosis and cryptococcosis), even in endemic areas1. In addition to the hypothesis that PCM may be underdiagnosed, this situation is likely due to the widespread use of sulfamethoxazole combined with trimethoprim (SMZ-TMP) in people living with HIV/AIDS (PLWHA), both for prophylaxis and for the treatment of other opportunistic diseases like cerebral toxoplasmosis and pneumocystosis1. When coinfection occurs, PCM typically presents with manifestations of both the acute/subacute and chronic forms, characterizing the disease as disseminated, with involvement of the lymph nodes, skin, and lungs (including atypical pulmonary changes such as cavitations and nodules). It may also present with hepatosplenomegaly, brain lesions, and lesions in other organs and tissues. Mixed presentations are more common in PLWHA with a T-CD4+ lymphocyte count <200 cells/mL2.
In this report, we describe a case of a patient with PLWHA who was severely immunocompromised and had disseminated PCM, including meningitis, but without pulmonary manifestations.
CASE REPORT
A 33-year-old man from Sao Paulo presented to the emergency room because of nausea and loss of appetite for 3 days. Over the previous 2 weeks, he had experienced asthenia, a strong, pulsating headache predominantly on the left side, sleepiness, and photophobia. He also reported weight loss of 30 kg over the past 4 months and diarrhea (without blood or mucus) for about a month, along with fever (unmeasured) and night sweats. He had been diagnosed with HIV infection in 2016 and had been taking tenofovir (TDF), lamivudine (3TC), darunavir, and ritonavir (DRV/r) irregularly. At the time, he was unemployed and had previously worked in a printing company; he denied any recent trips or agricultural practices. On initial examination, his condition was fair, but he appeared pale and dehydrated. Neurologically, he scored 14 on the Glasgow scale and was disoriented in time and space. His muscle strength was preserved, but he had a positive Hoffman reflex and left patellar hyperreflexia.
A computed tomography (CT) scan of the brain showed multiple intra-axial lesions, primarily in the basal nuclei, with no significant expansive effect and no contrast enhancement (Figure 1A). Magnetic resonance imaging (MRI) revealed leptomeningeal enhancement and confirmed the presence of multiple expansive lesions (Figures 2A and 2B). The results of the first lumbar puncture showed an opening pressure of 23 cm H2O, 8 cells/mm3 (95% lymphocytes), glycorrhachia of 51 mg/dL, and protein level of 80 mg/dL. Gram, India-ink, and Ziehl-Neelsen stains, the GeneXpert® MTB/RIF Ultra test, the latex test for cryptococcal antigen, polymerase chain reaction for Epstein-Barr virus, Herpes simplex, and Varicella zoster, bacterial cultures (aerobic bacteria), and the examination for neoplastic cells were all negative. The CD4+ cell count was 9 cells/mL, and the HIV viral load was 19,206 copies/mL. The IgG anti-toxoplasma antibody test was reactive in the serum. Given the severe immunodeficiency, neurotoxoplasmosis was considered the primary diagnostic hypothesis, with neurotuberculosis and primary CNS lymphoma as other differential diagnoses. Empirical treatment was started with pyrimethamine 50 mg/day, sulfadiazine 1,000 mg every 6 hours, and folinic acid 15 mg/day, along with reintroduction of the antiretroviral regimen (TDF+3TC+DRV/r). On the day 8 of treatment, dexamethasone (4 mg) was added every 8 hours.
Figure 1. Selected images from the patient’s computed tomography (CT) scans of the brain: 1A: Contrast-enhanced CT performed on admission: axial section showing rounded hypodense areas in the basal nuclei bilaterally, with no obvious enhancement or mass effect. 1B: Contrast-enhanced CT 2 weeks after admission: axial section showing reduction in previously described lesions. 1C: Contrast-enhanced CT 2 weeks after admission: axial section showing the appearance of a focal lesion in the right hemicerebellum with irregular enhancement and no obvious mass effect.
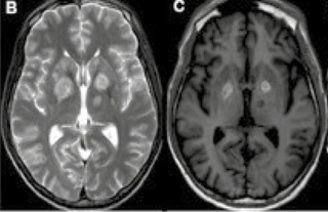
Figure 2. Selected images from magnetic resonance imaging (MRI) scans of the brain: 2A: T1 MRI without gadolinium, performed on admission: axial section shows rounded hypointense areas in the basal nuclei bilaterally, with no obvious mass effect. 2B: T2 sequence from the same exam: axial section shows a slight mass effect in the two main lesions located in the basal nuclei. 2C: MRI T1 without gadolinium, performed 3 weeks after the previous MRI: appearance of hypersignal in the previously described lesions of the basal nuclei, suggesting hemorrhagic content. 2D: T2-weighted magnetic resonance imaging: axial section showing reduction in the size and mass of the lesions in the basal nuclei.
The patient showed partial improvement in neurological symptoms, and a follow-up CT scan of the skull was performed after 14 days for control purposes (Figures 1B and 1C). Some lesions showed partial improvement, whereas others appeared, as shown in Figure 1C. The control MRI also showed a reduction in the size and perilesional edema of the lesions in the basal nuclei (Figures 2C and 2D) and a partial reduction in leptomeningeal enhancement, but also new lesions had appeared. The patient’s clinical course and new radiological findings suggested that cerebral toxoplasmosis was still the leading hypothesis, possibly associated with another granulomatous disease, such as tuberculosis or paracoccidioidomycosis, despite normal chest X-ray and CT findings. Given the presence of bilateral cervical and intraparotid lymphadenopathy (both non-palpable but with an apparently liquefied center) on the last brain CT scan, it was decided to perform a lymph node ultrasound-guided needle biopsy. The pathological examination of the lymph node specimen revealed a chronic granulomatous inflammatory reaction with areas of necrosis and numerous structures compatible with fungi, suggesting the presence of Paracoccidioides spp. due to its characteristic budding pattern shown by periodic acid-Schiff and Grocott staining. Ziehl-Neelsen staining was negative for acid-fast bacilli. Another biopsy of a small lesion on the hard palate also revealed fungal forms compatible with Paracoccidioides spp. Specific treatment with amphotericin B lipid complex (300 mg/day) was started immediately, while sulfadiazine, pyrimethamine, folinic acid, and dexamethasone were continued. After 14 days, a new cerebrospinal fluid (CSF) sample showed 3 cells/mm3, glycorrhachia of 89 mg/dL, a protein level of 44 mg/dL, and a lactate level of 22 mg/dL. The fungal test was negative, but the immunological reaction by ELISA for PCM was positive in that CSF sample.
The patient developed several complications and infections during a prolonged hospital stay, including pulmonary thromboembolism and episodes of sepsis caused by Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus haemolyticus. He also developed severe rhinosinusitis with septal perforation. A biopsy isolated Candida glabrata, which was treated with extended bilateral maxillectomy. Finally, the patient developed COVID-19 in-hospital, which led to severe respiratory failure and death after 47 days of hospitalization. Autopsy was not performed.
DISCUSSION
PCM infection starts in the lungs and can spread to other organs and tissues via blood and/or lymphatic pathways. CNS involvement occurs through this dissemination4-5 and is described in up to 27% of PCM cases. It is a severe, potentially disabling, and even fatal form of PCM. NPCM primarily involves the brain parenchyma, with secondary involvement of the meninges5.
The clinical manifestations depend on the location of the lesions. The most common manifestations are headache, focal signs, seizures, intracranial hypertension, ataxia, and neurocognitive alterations. Spinal cord involvement is rare5. The most common non-neurological manifestations in patients with NPCM are respiratory symptoms, which occur in over 85% of cases and often precede neurological symptoms5. Active radiological investigation of the lungs is justified to aid diagnosis, as the lungs are the most affected organ6. However, in our case, the chest CT scan was normal. In contrast, NPCM in patients with severe immunosuppression may be associated with acute and severe symptoms of disseminated infection involving organs like the reticuloendothelial system, lungs, skin, and mucous membranes, but with no prominent neurological symptoms at all7,8.
NPCM typically involves the supratentorial compartment in 67% of cases, with a particular focus on the cerebral hemispheres7. MRI is more sensitive than CT for detecting small and/or posterior fossa lesions6, but in this case, CT was able to adequately identify both initial and new lesions.
Differential diagnoses vary between immunocompetent (neoplasms, tuberculomas, or cryptococomas) and immunocompromised patients (cerebral toxoplasmosis, tuberculosis, and lymphomas)6-9. The management of expansive brain lesions in patients with PLWHA is well-established, with presumptive treatment for cerebral toxoplasmosis (just as it was our first hypothesis). Lesions secondary to this opportunistic disease usually improve within 10-14 days of empirical treatment10.
Identification of Paracoccidioides spp. is necessary for diagnostic confirmation and is achieved through histopathological examination (by micromorphological identification of the agent) or by culture of tissue fragments from other affected sites7 (in our case, cervical lymph nodes and palate). Analysis of CSF is of limited value in NPCM6: it usually has a clear appearance and normal opening pressure, and it typically shows non-specific alterations that hardly define the diagnosis. Direct testing for the fungus or its isolation in culture usually produces negative results3. The detection of antibodies in CSF is highly sensitive and takes less time than antigen detection, although cross-reactions with other fungal agents can occur, especially histoplasmosis3. The detection of the glycoprotein antigens gp43 and gp70 by inhibition ELISA can contribute to specific diagnosis3. The measurement of anti-PCM antibodies in the blood often gives false-negative results, especially in cases of very localized lesions and in immunocompromized hosts1. No serological tests were performed for this patient.
There is no solid evidence that PCM is resistant to azole derivatives (fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole), sulfamide (cotrimoxazole, sulfadiazine), and amphotericin B (deoxycholate, lipid complex, and liposomal)1. The most commonly used agents in clinical practice are itraconazole, the SMZ-TMP association, and amphotericin B. For less severe cases, oral itraconazole (for an average of 12 months) is the recommended treatment because of its high efficacy and safety. SMZ-TMP is another option, possibly even when there is CNS involvement1. However, in severe cases of disseminated PCM (including NPCM), the recommended treatment consists of two phases: a first induction phase with intravenous drugs (most commonly amphotericin B) maintained until laboratory parameters return to normal and the clinical picture is controlled; and a second maintenance phase with oral drugs for an indefinite period, also depending on the clinical response1,11,12. A neurosurgical approach may be necessary in patients with hydrocephalus secondary to brain lesions, in the form of ventriculoperitoneal or external ventricular shunt - resection of mass-effect lesions is infrequent12.
CONCLUSION
HIV/PCM co-infection can lead to death, but this was not the direct cause of the unfavorable outcome in our patient. This report demonstrates the difficulty of establishing a definitive diagnosis of NPCM in patients with PLWHA because the clinical manifestations are common to other more frequent diseases (especially neurotoxoplasmosis), imaging is heterogeneous, the performance of immunological tests is limited, and it is technically difficult to obtain samples for histopathological analysis. This warrants a concomitant investigation into other affected sites.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Plank R, Dean D. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2000;2(10):1265-76. DOI: 10.1016/s1286-4579(00)01280-6
2. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços. Guia de Vigilância em Saúde: volume único, Capítulo 10 – Leptospirose [Internet]. 3ª ed. Brasília: MS; 2019; [cited 2024 Jan 31]. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_3ed.pdf
3. Hotez PJ, Fujiwara RT. Brazil’s neglected tropical diseases: an overview and a report card. Microbes Infect. 2014;16(8):601-6. DOI: 10.1016/j.micinf.2014.07.006
4. Martins MHDM, Spink MJP. Human leptospirosis as a doubly neglected disease in Brazil. Cien Saude Colet. 2020;25(3):919-928. DOI: 10.1590/1413-81232020253.16442018
5. Ministério da Saúde (BR). Sistema de Informação de Notificação de Agravos (SINAN) [Internet]. Brasília: MS; 2007; [cited 2022 Feb 04]. Available from: https://datasus.saude.gov.br/
6. Togal T, Sener A, Yucel N, Demirbilek S, Akgun FS, Aydogan M, et al. Intensive care of a weils disease with multiorgan failure. J Clin Med Res. 2010;2(3):145-9. DOI: 10.4021/jocmr2010.05.302w
7. de Brito T, Silva AMGD, Abreu PAE. Pathology and pathogenesis of human leptospirosis: a commented review. Rev Inst Med Trop Sao Paulo. 2018;60:e23. DOI: 10.1590/s1678-9946201860023
8. Medeiros FR, Spichler A, Athanazio DA. Leptospirosis-associated disturbances of blood vessels, lungs and hemostasis. Acta Trop. 2010;115(1-2):155-62. DOI: 10.1016/j.actatropica.2010.02.016
9. Pereira JCB, Grijó A, Mackay MCB, Vitral BG. Leptospirose pulmonar. Rev Port Pneumol. 2007;13(6):827-39. DOI: 10.1016/S0873-2159(15)30378-0
10. Marotto PC, Ko AI, Murta-Nascimento C, Seguro AC, Prado RR, Barbosa MC, et al. Early identification of leptospirosis-associated pulmonary hemorrhage syndrome by use of a validated prediction model. J Infect. 2010;60(3):218-23. DOI: 10.1016/j.jinf.2009.12.005
11. Park JA. Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. Int J Mol Sci. 2021;22(2):793. DOI: 10.3390/ijms22020793
12. Daher EDF, Abreu KLS de, Silva Junior GB da. Insuficiência renal aguda associada à leptospirose. Braz J Nephrol. 2010;32(4):408-15. DOI: 10.1590/S0101-28002010000400010
13. Chancharoenthana W, Leelahavanichkul A, Schultz MJ, Dondorp AM. Going Micro in Leptospirosis Kidney Disease. Cells. 2022;11(4):698. DOI: 10.3390/cells11040698
14. Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2(4):739-44. DOI: 10.2215/CJN.00680207
15. Maxwell RA, Bell CM. Acute Kidney Injury in the Critically Ill. Surg Clin North Am. 2017;97(6):1399-418. DOI: https://doi.org/10.1016/j.suc.2017.07.004
16. Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346(5):305-10. DOI: 10.1056/NEJMoa010877
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















