Osteolytic lesions in a patient living with HIV
DOI:
https://doi.org/10.5935/2764-734X.e20250353Keywords:
Headings: AIDS-related Opportunistic Infections, Kaposi’s Sarcoma, Osteoarticular Tuberculosis, Human Herpesvirus 8, Case reportAbstract
This case report describes a 22-year-old HIV-infected male patient with pulmonary and skin complaints, whose initial computed tomography images showed lytic lesions on the sternum, scapula, and vertebral bodies as well as on the skullcap, iliac bone, sacrum, humerus, tibia, and femur, immediately raising the suspicion of a metastatic neoplasm or multiple myeloma. Pulmonary tuberculosis (with preserved sensitivity to rifampicin) was diagnosed through the sputum, and a skin biopsy confirmed the diagnosis of Kaposi's sarcoma. However, the bone biopsy of the sternum was inconclusive - although it ruled out any type of neoplastic infiltration, it was unable to confirm tuberculosis or sarcoma in that topography. The patient ran away from hospital before the investigation progressed toward a definitive diagnosis, but a brief review of the literature highlights the possibility of opportunistic etiologies causing osteolytic lesions in HIV/AIDS patients.
Downloads
INTRODUCTION:
In patients with osteolytic lesions, the initial diagnostic hypothesis is usually metastatic or primary neoplastic diseases, especially multiple myeloma1,2. This case report refers to a patient with HIV/AIDS and disseminated osteolytic lesions who had a confirmed diagnosis of pulmonary tuberculosis and disseminated Kaposi’s sarcoma (KS) (skin and stomach), without any evidence of other associated neoplastic processes. This study aimed to highlight the particularities and complexity of this radiological finding in this group of immunocompromised patients.
CASE REPORT:
A 22-year-old homeless man from Sao Paulo presented to the emergency room complaining of a chronic cough and progressive dyspnea for a year. This was associated with an unmeasured fever and chills as well as unquantified weight loss. He reported significant worsening of his respiratory symptoms in the last 2 weeks, with a more productive cough and dyspnea on minimal effort. He denied any type of pain. He also reported the presence of bullous lesions in the inguinal region for approximately 6 months, with a progressive increase in their extent. The patient had known he was HIV-positive since 2021 and had maintained a regular antiretroviral treatment regimen until November 2022, when he stopped taking the medication. The latest data extracted from the Laboratory Test Control System of the Department of HIV/AIDS, Tuberculosis, Viral Hepatitis and Sexually Transmitted Infections (SISCEL; available at https://siscel.aids.gov.br), documented a viral load of 4,808 copies/mL and CD4 of 356 cells/mm3, both from the beginning of 2023.
On physical examination, the patient was ruddy, hydrated, afebrile, normotensive, normocardic, and tachypneic, with an oxygen saturation of 98% in room air. Asymmetrical edema was noted in the lower limbs. It was more pronounced on the right side, with hardened, infiltrative violaceous lesions on the skin of the inguinal and medial regions of the thighs bilaterally, some of them ulcerated, with nodulations and hyperemia (Figure 1). Pulmonary auscultation revealed a reduced vesicular murmur in both bases. Computed tomography (CT) of the chest revealed pulmonary nodules suggestive of hematogenous distribution, associated with bilateral pleural effusion (Figure 2A). Sputum samples were collected, which tested positive for acid-fast bacilli, and the molecular test for M. tuberculosis was reactive with preserved sensitivity to rifampicin, establishing the diagnosis of pulmonary tuberculosis. Analysis of the pleural fluid revealed an uncomplicated bilateral exudate, with all microbiological tests negative. Histological analysis of the pleural biopsy documented mild chronic pleuritis with fibrosis, with no evidence of malignancy or granulomas. Bilateral drainage was performed to relieve the respiratory symptoms. Moreover, another finding on the chest CT revealed the presence of lytic lesions on the sternum, scapula, and vertebral bodies (Figure 2B), while other tests to determine a more complete staging of a possible metastatic neoplasm showed further lytic lesions, on the skullcap, iliac bone, sacrum, humerus, tibia, and femur (Figures 3 and 4). Serum beta-2 microglobulin levels were within the normal range, and serologies for syphilis, HTLV 1, and 2 as well as hepatitis B and C were non-reactive. A skin biopsy of the lesions on the right thigh revealed atypical vascular proliferation with positive immunohistochemistry for HHV8, thus confirming the diagnosis of KS. Furthermore, upper gastrointestinal endoscopy, performed for staging purpose, showed violaceous lesions in the esophagus, stomach, and duodenum (Figure 5), the biopsies of which showed the same pattern of atypical vascular proliferation and positive HHV8. Surgical bone biopsy of the lytic lesion on the sternum was performed, but its histological analysis was non-specific, showing no malignancy or granulomas, with all three hematopoietic series under an age-appropriate profile. Microbiological and immunohistochemical tests on the bone samples were all negative for tuberculosis, fungi, and HHV8. Treatment for tuberculosis and chemotherapy for KS were started in the second week of hospitalization and led to relative clinical improvement. However, the patient ran away from hospital on the 63rd day, before the already scheduled positron emission tomography (PET-CT) scan and a new bone biopsy (at a different site) were done to define the diagnosis of the osteolytic lesions.
Figure 1. Appearance of skin lesions in the genital region and lower limbs.
Figure 2. A - chest tomography showing multiple solid nodules of random distribution scattered throughout both lungs, suggestive of a hematogenous distribution pattern, associated with large bilateral pleural effusion. B - lytic lesions in the vertebral body and sternum (arrow).
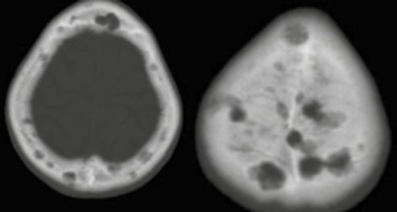
Figure 3. Osteolytic lesions in the cranial vault.
Figure 4. Lytic lesions in the humerus (a), scapula and humerus (b), ilium and sacrum (c), tibia (d) and femur (e).
Figure 5. Upper digestive endoscopy showing the presence of violaceous lesions in the esophagus, stomach and duodenum.
DISCUSSION:
Differential diagnoses of osteolytic lesions in patients living with HIV/AIDS (PLWHA) should be evaluated with a different level of suspicion from the nonimmunosuppressed population3. Taking this case report into account, for example, tuberculosis and KS can mimic the radiological findings most commonly associated with neoplasms as well as present similar and confusing clinical and laboratory manifestations.
From a radiological perspective, metastatic lesions of solid tumors usually present as well-defined areas of bone destruction, revealing marked translucency in the images, even during the early stages of the disease1. Conversely, multiple myeloma is characterized by osteolytic lesions, often multiple and in various topographies, with typical radiographic changes showing areas of demineralization associated with a cuff pattern in the bones4. KS may also manifest as multifocal osteolytic lesions, although it most often affects the long bones and vertebrae5-8. Bone tuberculosis has a greater predilection for the spine, but can also involve other bones and joints9-10.
A higher incidence of multiple myeloma in PLWHA is a controversial issue. In an analysis of a North American database, myelomas accounted for 49 cases out of a total of 2,552 patients with non-AIDS-defining cancers11. In another single-center retrospective series from South Africa, 16 of the 89 patients with multiple myeloma were HIV-positive and, of these, 11 had osteolytic lesions12. There is, however, a statistically significant difference in relation to the younger age of this population at the time of diagnosis of myeloma, which can happen at any stage of HIV infection, whether early on or after decades of chronic evolution11.
The presentation of disseminated KS through osteolytic lesions, although uncommon, has been described in PLWHA, with or without the presence of tumor masses in contiguous soft tissues5-8. Biopsies in these cases (puncture and open) are usually fundamental in establishing this diagnosis5,8,13. Bone involvement in coinfection of tuberculosis with HIV is more likely to cause erosions that characterize spondylitis or Pott’s disease9-10, whereas isolated multifocal lytic lesions are much less commonly described14. Other differential diagnoses of osteolytic lesions caused by opportunistic infections in PLWHA are secondary syphilis15-16 and bacillary angiomatosis, considering that the macroscopic appearance of skin lesions in the latter is very similar to KS17-18.
Herein, it was not possible to establish a definitive diagnosis, even through bone biopsy. Although frustrating, it is not uncommon for bone biopsies to be undefined in this context19, while in more than 50% of cases of osteolysis, biopsy is not even performed7. Regardless, our patient’s sternal biopsy made it possible to rule out the diagnosis of any metastatic neoplasm and of myeloma proper (all the more so given the normal serum beta 2 microglobulin dosage), emphasizing that the diagnoses of tuberculosis and KS were definitive, but at sites other than the bone. Also notable is the total absence of pain reported by the patient and the satisfactory clinical evolution promoted by the treatment. Unfortunately, with the patient leaving the hospital, it was not possible to perform further procedures aimed at defining this diagnostic impasse.
CONCLUSION:
This case report highlights the importance of broadening the range of possible etiologies for osteolytic lesions in PLWHA. The clinical and radiological manifestations of infections can overlap with those of malignancies, which makes clinical management difficult. Bone biopsies are essential to clarify the diagnosis in this context, but they can be inconclusive. The concomitant investigation of other affected regions and organs can help in diagnostic reasoning and guide conduct in similar cases.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev. 2021 Jul 1;101(3):797-855. DOI: 10.1152/physrev.00012.2019
2. Subramanian S, Viswanathan VK. Lytic bone lesions. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2024. [Accessed 2024 Sep 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539837
3. Tehranzadeh J, Ter-Oganesyan RR, Steinbach LS. Musculoskeletal disorders associated with HIV infection and AIDS: Part I: infectious musculoskeletal conditions. Skeletal Radiol. 2004 May;33(5):249-59. DOI: 10.1007/s00256-004-0764-z
4. Rajkumar SV. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. 2024 Sep;99(9):1802-24. DOI: 10.1002/ajh.27422
5. Yergiyev O, Mohanty A, Curran-Melendez S, Latona CR, Bhagavatula R, Greenberg L, et al. Fine-needle aspiration cytology of disseminated Kaposi sarcoma of the bone in an AIDS patient. Acta Cytol. 2015;59(1):113- 7. DOI: 10.1159/000369855
6. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004 Apr;33(4):241-3. DOI: 10.1007/s00256-003-0732-z
7. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz L. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007 Mar 15;109(6):1040-52. DOI: 10.1002/cncr.22500
8. Bell BM Jr, Syed A, Carmack SW, Thomas CA, Layton KF. Disseminated Kaposi sarcoma with osseous metastases in an HIV-positive patient. Proc (Bayl Univ Med Cent). 2016 Jan;29(1):52-4. DOI: 10.1080/08998280.2016.11929358
9. Hodkinson B, Osman N, Botha-Scheepers S. HIV Infection and Osteoarticular Tuberculosis: Strange Bedfellows. Case Rep Rheumatol. 2016;2016:5718423. DOI: 10.1155/2016/5718423
10. Pigrau-Serrallach C, Rodríguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013 Jun;22(Suppl 4):556-66. DOI: 10.1007/s00586-012-2331-y
11. Shiels MS, Althoff KN, Pfeiffer RM, Achenbach CJ, Abraham AG, Castilho J, et al. Engels EA; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiologic Databases to Evaluate AIDS (IeDEA). HIV Infection, Immunosuppression, and Age at Diagnosis of Non - AIDS-Defining Cancers. Clin Infect Dis. 2017 Feb 15;64(4):468-75. DOI:10.1016/S2352-3018(20)30118-1
12. De Groot JJB, Webb MJ, Raubenheimer JE, Struwig MC, Louw VJ. Concomitant HIV infection in newly diagnosed multiple myeloma patients is hard to recognise and should be tested for routinely in areas of high endemicity. S Afr Med J. 2017 Aug 25;107(9):781-7. DOI:10.7196/SAMJ.2017.v107i9.12360
13. Connolly SP, McGrath J, Sui J, Muldoon EG. Rare, disseminated Kaposi sarcoma in advanced HIV with high-burden pulmonary and skeletal involvement. Rare, disseminated Kaposi sarcoma in advanced HIV with high-burden pulmonary and skeletal involvement. BMJ Case Rep. 2021 Dec 1;14(12):e245448. DOI: 10.1136/bcr-2021-245448
14. Mellat-Ardakani M, Ghiasvand F, Nezhad MH, Salahshour F, SeyedAlinaghi S. Multifocal Osteolytic Lesions in Skull Bone with Mycobacterium Tuberculosis: A Case Report. Infect Disord Drug Targets. 2021;21(5):e270421187878. DOI: 10.2174/18715265 20999201111200140
15. Colquhoun M, Kirresh O, Keikha M, Haddow L. Osteolytic lesions as the sole presenting feature of secondary syphilis. BMJ Case Rep. 2021 Jun 22;14(6):e242814. DOI:10.1136/bcr-2021242814
16. Park KH, Lee MS, Hong IK, Sung JY, Choi SH, Park SO, et al. Bone involvement in secondary syphilis: a case report and systematic review of the literature. Sex Transm Dis. 2014 Sep;41(9):532-7. DOI: 10.1097/OLQ.0000000000000164
17. Braekeveld P, Verstraete K, Deprest K, Van Hecke E, Kunnen M. Bacillary Angiomatosis in a Patient with AIDS - case 1717 [Internet]. EURORAD 2022 (8 oct). Accessed in 2025 January 10. Available from: www.eurorad.org/case/1717. DOI: 10.1594/EURORAD/CASE.1717
18. Baron AL, Steinbach LS, LeBoit PE, Mills CM, Gee JH, Berger TG. Osteolytic lesions and bacillary angiomatosis in HIV infection: radiologic differentiation from AIDS-related Kaposi sarcoma. Radiology. 1990 Oct;177(1):77-81. DOI: 10.1148/radiology.177.1.2399342
19. Murugan S. Multiple osteolytic lesions in a 14-year-old boy with HIV disease. Indian J Sex Transm Dis AIDS. 2015 Jan/Jun;36(1):92-4. DOI: 10.4103/2589-0557.156747
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















