Expansive brain lesion in a patient with AIDS
DOI:
https://doi.org/10.5935/2764-734X.e20250255Keywords:
AIDS-Related Opportunistic Infections, Tuberculoma, Intracranial, Toxoplasmosis, cerebral, Case reportAbstract
A cerebral tuberculoma is a potential complication of disseminated tuberculosis in people living with HIV/AIDS, especially in developing countries. We report the case of a 52-year-old woman with advanced HIV disease and confirmed pulmonary tuberculosis, who presented with mental confusion, syncope, and a multilobulated intracranial tumor. She exhibited clinical and radiological worsening after 3 months of treatment (already in the maintenance phase), which resulted in therapeutic doubts: cerebral toxoplasmosis was the main differential diagnosis and empirical treatment was initiated. We also decided to reinitiate the complete tuberculosis regimen and add corticosteroids to it. Examination of a second sample of cerebrospinal fluid showed acid-fast bacilli; however, a stereotactic biopsy did not show any etiologic agent. After a prolonged hospital stay of 2 months, the patient was discharged to an outpatient clinic where she followed up for almost 2 years presenting evident clinical improvement, but with only partial improvement in terms of neuroimaging.
Downloads
INTRODUCTION
Neurological manifestations occur in approximately 1% of tuberculosis cases, the most common form being meningitis1. However, expansive lesions in the central nervous system (CNS) can also occur in neurotuberculosis, a condition that is particularly challenging to diagnose in people living with HIV/AIDS (PLWHA) due to the presence of key differential diagnoses such as cerebral toxoplasmosis, primary CNS lymphoma, chagoma, syphilitic gumma, and pyogenic abscess1-3.
In this report we present the case of a patient with AIDS presenting a sizeable brain lesion suggestive of tuberculoma and discuss the difficulties in diagnosing and treating this lesion.
CASE REPORT
A 52-year-old woman with HIV, whose condition had not been medically followed up for more than 10 years, visited an infectious disease outpatient clinic complaining of weight loss, respiratory symptoms, and shingles infection. At the time, the physician prescribed acyclovir and restarted antiretroviral therapy (ART) with dolutegravir, tenofovir, and lamivudine, but did not investigate further. Moreover, the patient had an HIV viral load of 320,000 copies/mL and a CD4+ count of 88 cells/mm3.
Approximately 15 days after initiating ART, her respiratory symptoms worsened and she presented mental confusion and syncope; she was then admitted to another department. There, she was diagnosed with pulmonary tuberculosis after testing positive using sputum smear microscopy. Treatment with rifampicin (R), isoniazid (H), pyrazinamide (Z), and ethambutol (E) was initiated, and the same ART regimen was continued; however, with a double dose of dolutegravir because of the use of rifampicin. There are no reports of the use of corticosteroids at this time.
To investigate the neurological condition, a CT scan of the skull was performed, which showed a multilobulated tumor lesion located in the left frontal parasagittal region that measured 3.6 × 1.9 cm in its longest dimensions comprising juxtaposed, hypodense oval images with ring-shaped contrast enhancement. Cerebrospinal fluid (CSF) analysis showed 1 cell/mm3, and protein levels of 100 mg/dL, negative results for sputum smear microscopy. The patient was discharged after 2 months with continuation of rifampicin and isoniazid (RH), with no documented record of the diagnostic hypotheses for her neurological condition.
Fifteen days after starting the maintenance phase of tuberculosis therapy and after about 90 days of using ART, the patient visited our hospital with mental confusion, diplopia, and loss of overall strength. At this time, the HIV viral load was 758 copies/mL and CD4+ counts were 124 cells/mm3. Another CT scan of the skull was performed, which showed an increase in the size of the previously described lesion, now measuring 5.2 × 2.7 cm, and contrast enhancement was maintained. Another CSF sample was collected that tested positive for acid-alcohol resistant bacilli (BAAR), despite the negative result of the GeneXpert Ultra® test for Mycobacterium tuberculosis. We then decided to continue RH and initiate empirical treatment for cerebral toxoplasmosis.
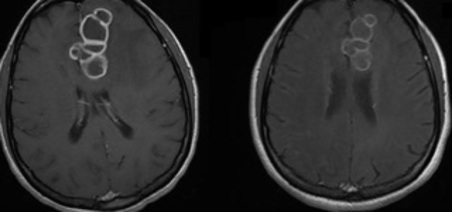
Magnetic resonance imaging (MRI) facilitated better characterization of the multilobulated contour by revealing a thick capsule and central hypointensity on the T2 sequence (Figure 1A). This finding helped us to once again prioritize the hypothesis of cerebral tuberculoma; we therefore decided to reinitiate the complete RHZE regimen without suspending the toxoplasmosis treatment, which was continued for 42 days given the possibility of concomitant neurological infections. To clarify this, we requested a stereotactic brain biopsy and the histological study showed chronic inflammatory changes in the glial environment with no evidence of malignancy or granulomas. In addition, bacilloscopy, GeneXpert® for M. Tuberculosis, fungal testing, and immunohistochemistry results were all negative, including the latest results of the respective cultures.
Figure 1. T1-weighted magnetic resonance imaging (MRI) in post-gadolinium axial slices). 1A- MRI performed at the time of hospitalization (after 90 days of ART and 60 days of RHZE); 1B- another exam performed after 120 days of RHZE; 1C - the last MRI performed on an outpatient basis, after almost 2 years.
Although we were unable to establish a definitive microbiological and histological diagnosis of tuberculoma, the patient’s neurological symptoms gradually improved. Two months after the reinitiation of RHZE, plus 1 month of RH and the use of corticosteroids throughout these 3 months, an additional MRI showed a reduction in the volume of the lesion and smoothing of its edges (Figure 1B). At this time, the patient still had occasional focal motor seizures (regulated using sodium valproate), which contributed to a prolonged hospital stay of 70 days.
She was re-evaluated as an outpatient after almost 2 years of anti-tuberculosis treatment and ART with a double dose of dolutegravir; although there was evident neurological improvement, radiological improvement was only partial (Figure 1C). At that time, her HIV viral load was 158 copies/mL and her CD4+ counts were 560 cells/mm3. Figure 2 shows a timeline of the main events described in this report, for educational purposes.
Figure 2. Timeline of the main events of the case. Legend: VL = viral load; ART = antiretroviral therapy; R + H = rifampicin + isoniazid.
DISCUSSION
Neurotuberculosis manifests clinically as fever, headache, mental confusion, convulsions, cranial nerve paralysis, or other focal neurological signs. Imaging exams may show signs of meningitis, vasculitis, hydrocephalus, and/or expansive lesions2-4. However, the existence of more than one clinical syndrome caused by M. tuberculosis5,6 is not uncommon, as in the case of this patient who had diffuse meningoencephalitis in addition to an expansive lesion.
Biopsy has emerged as the gold standard diagnostic method for CNS tuberculomas7. However, this procedure is invasive, has variable sensitivity, and samples may not be easily accessible. The biopsy was inconclusive in the present case, either because of unrepresentative biopsy sampling or because it was performed after a long period of treatment (112 days). Because the biopsy was uninformative, we decided to complete the full treatment for neurotoxoplasmosis simultaneously with other procedures, considering the risk-benefit of this measure and the epidemiology of the disease in PLWHA.
In the context of uncertainty about a diagnosis of tuberculoma, a study published in 2020 proposed a score that combines radiological and clinical-epidemiological aspects to facilitate diagnostic reasoning and allow early treatment8. This scoring system (SVIMS criteria) uses major and minor criteria and the analysis of the total score has good sensitivity and specificity8. Our patient presented 2 major (the radiological finding of lobulated lesions >20 mm with hypointensity in T2) and six minor criteria, including significant perilesional edema, more than one lesion in a conglomerate, and the presence of confirmed tuberculosis elsewhere8.
The treatment of cerebral tuberculoma involves a combination of pharmacological interventions and, in selected cases, surgery8-10. The RHZE regimen comprises the first-line treatment; however, its duration is controversial, varying between 9 and 24 months10. The Brazilian guidelines recommend the usual intensive phase of 2 months with RHZE followed by a further 10 months of RH1. However, it is crucial to consider extending the intensive phase in cases of high bacillus load and failure of clinical/radiological response, considering the joint action of four anti-tuberculosis drugs instead of two9,10.
Lesions larger than 2.5 cm in diameter have a poor prognosis and their treatment is prolonged (over 24 months) if they are not surgically removed8. In our case, we decided not to operate on the patient given her satisfactory clinical response. Surgical intervention should also be considered in cases of cranial hypertension, significant mass effect, or hydrocephalus, and ventriculoperitoneal shunting may be necessary8.
Corticosteroids should be administered during the first 6 weeks or until a significant clinical improvement is observed, as they reduce inflammation and edema associated with the lesions11. Our patient only received corticosteroids at a later stage due to clinical and radiological worsening, and the benefit of their use at this stage is controversial according to reports11.
The possibility of a patient co-infected with tuberculosis and HIV developing immune reconstitution syndrome warrants two additional comments. The first refers to the sequence of treatments. The guidelines recommend introducing ART only after a minimum of 15 days of initiating tuberculosis treatment1. This did not happen in our case. It would have been prudent to prioritize the investigation of the initial complaints (which resulted in a confirmed diagnosis of tuberculosis soon after) rather than reintroducing ART immediately upon the patient’s first visit. The second comment refers to the possibility that the patient’s clinical and radiological worsening is attributable precisely because of this sequence, i.e., due to the development of the immune reconstitution syndrome. The development of paradoxical reactions in the CNS should always be considered upon lesion regrowth after the initiation of treatment and in the presence of immune recovery markers, namely an increase in the CD4+ cell counts and a significant decrease in the HIV viral load11,12. In our opinion, the worsening of the brain lesion revealed upon imaging examination was probably due to the progression of the infection, because BAAR was detected in the CSF, a finding that is not compatible with a paradoxical reaction12.
CONCLUSION
Tuberculomas should be included in the differential diagnosis of brain lesions in PLWHA. This report demonstrates that establishing an etiological diagnosis of tuberculoma can be challenging, even with a brain biopsy, and that the use of a scoring system comes as an aid in this context. Prolonging the treatment and using corticosteroids concomitantly are two complementary and effective strategies that should be considered in similar cases.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de Recomendações para manejo da infecção pelo HIV em adultos. 1ª ed. Brasília: Ministério da Saúde; 2024. [Acesso 2025 fev 12]. Disponível em: https://www.gov.br/aids/pt-br/central-de-conteudo/pcdts/pcdt_hiv_modulo_1_2024.pdf
2. Vidal JE, Cimerman S, da Silva PR, Sztajnbok J, Coelho JF, Lins DL. Tuberculous brain abscess in a patient with AIDS: case report and literature review. Rev Inst Med Trop Sao Paulo. 2003 Mar-Apr;45(2):111-4. DOI: 10.1590/S0036-46652003000200013
3. Park M, Gupta RK. Central Nervous System Mycobacterium Infection: Tuberculosis and Beyond. Neuroimaging Clin N Am. 2023 Feb;33(1):105-24. DOI: 10.1016/j.nic.2022.07.006
4. Bernaerts A, Vanhoenacker FM, Parizel PM, Van Goethem JW, Van Altena R, Laridon A, et al. Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur Radiol. 2003 Aug;13(8):1876-90. DOI: 10.1007/s00330-002-1608-7
5. Marais S, Pepper DJ, Marais BJ, Török ME. HIV-associated tuberculous meningitis--diagnostic and therapeutic challenges. Tuberculosis (Edinb). 2010 Nov;90(6):367-74. DOI: 10.1016/j.tube.2010.08.006
6. Parry AH, Wani AH, Shaheen FA, Wani AA, Feroz I, Ilyas M. Evaluation of intracranial tuberculomas using diffusion-weighted imaging (DWI), magnetic resonance spectroscopy (MRS) and susceptibility weighted imaging (SWI). Br J Radiol. 2018 Nov;91(1091):20180342. DOI: 10.1259/bjr.20180342
7. Goyal V, Elavarasi A, Abhishek, Shukla G, Behari M. Practice Trends in Treating Central Nervous System Tuberculosis and Outcomes at a Tertiary Care Hospital: A Cohort Study of 244 Cases. Ann Indian Acad Neurol. 2019 Jan-Mar;22(1):37-46. DOI: 10.4103/aian.AIAN_70_18
8. Vemula RC, Prasad BCM, Koyalmantham V, Hanu G. Role of Surgery in Intracranial Tuberculomas and Proposal of a Novel Diagnostic Criteria for Diagnosis (Sri Venkateswara Institute of Medical Sciences Criteria). World Neurosurg. 2020 Jun;138:e52-e65. DOI: 10.1016/j.wneu.2020.01.179
9. Nair BR, Rajshekhar V. Factors Predicting the Need for Prolonged (>24 Months) Antituberculous Treatment in Patients with Brain Tuberculomas. World Neurosurg. 2019 May;125:e236-e247. DOI: 10.1016/j.wneu.2019.01.053
10. Marais S, Van Toorn R, Chow FC, Manesh A, Siddiqi OK, Figaji A, et al; Tuberculous Meningitis International Research Consortium. Management of intracranial tuberculous mass lesions: how long should we treat for? Wellcome Open Res. 2019 Oct 31;4:158. DOI: 10.12688/wellcomeopenres.15501.2
11. Donovan J, Bang ND, Imran D, Nghia HDT, Burhan E, Huong DTT, et al; ACT HIV Investigators. Adjunctive Dexamethasone for Tuberculous Meningitis in HIV-Positive Adults. N Engl J Med. 2023 Oct 12;389(15):1357-67. DOI: 10.1056/NEJMoa2216218
12. Vidal JE, Cimerman S, Schiavon Nogueira R, Bonasser Filho F, Sztajnbok J, da Silva PR, et al. Paradoxical reaction during treatment of tuberculous brain abscess in a patient with AIDS. Rev Inst Med Trop Sao Paulo. 2003 May-Jun;45(3):177-8. DOI: 10.1590/S0036-46652003000300012
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















