Cryptococcus gattii infection mimicking neurocysticercosis
DOI:
https://doi.org/10.5935/2764-734X.e20250458Keywords:
Meningitis, Cryptococcal, Cryptococcus gattii, Neurocysticercosis, Case ReportsAbstract
Cryptococcosis is a systemic mycosis caused by fungi of the genus Cryptococcus, with Cryptococcusneoformans and Cryptococcus gattii being the main species of medical importance. Although they share similarities, they show clinical and pathogenic differences due to structural and immunological variations and distinct mechanisms of evasion of the host response. We report the case of a 44-year-old woman with no known immunosuppression. She was initially referred for suspected neurocysticercosis and was nevertheless diagnosed with disseminated cryptococcosis caused by C. gattii, characterized by multiple pseudocysts in the central nervous system, a large pulmonary cryptococcoma, and an ulcerated skin lesion on the shoulder. The treatment included prolonged antifungal therapy, a lumboperitoneal shunt, resection of the lung mass, and corticosteroid therapy. After a lengthy hospital stay, the patient improved clinically and was discharged; however, she had neurological recurrences that required further hospitalizations, later evolving to death.
Downloads
Introduction
Cryptococcosis is a systemic mycosis that has a major impact on health worldwide. It is caused by encapsulated fungi of the genus Cryptococcus that are present globally in the environment. It is estimated that approximately 1.6 million annual deaths are related to the disease worldwide, especially among patients living with HIV/AIDS1.
There are two complexes of pathogenic species2: C. neoformans and C. gattii, both capable of causing serious invasive diseases. C. neoformans is a ubiquitous fungus and the main agent of cryptococcosis, especially in immunosuppressed individuals and those living with HIV, making it an opportunistic infection. C. gattii manifests more frequently as a primary infection in patients without known immunosuppression and poses a greater risk of unfavorable progression due to neurological complications, longer treatment times, and neurosurgical interventions3.
We report the case of a woman without known immunosuppression who had disseminated cryptococcosis caused by C. gattii, and whose clinical and surgical management was challenging.
CASE REPORT
This case was of a 44-year-old female patient, an administrator, previously healthy and with no history of immunosuppression, who was referred to our department in July 2023. Her condition had initiated 3 months earlier with a headache unrelieved by anti-inflammatory drugs, which became holocranial, intense, continuous, and accompanied by auditory hallucinations and memory loss. There were no associated febrile episodes; however, the patient developed asthenia and disorientation and became dependent on help for daily activities. Along with the neurological condition, an ulcerated and painless skin lesion appeared on the right shoulder. The week before she was admitted, she had diplopia and drowsiness and was assessed by a neurologist who ordered a nuclear magnetic resonance of the skull, described as “multiple cystic lesions in the brain suggestive of neurocysticercosis in the vesicular stage.” The patient had a history of smoking and controlled hypertension, with no other comorbidities. She denied previous exposure to animals (including birds), recent traveling, or activities in the forest and rural environments.
She was admitted in good general condition; she was clinically stable but disoriented in time and space; physical examination showed convergent strabismus in the left eye, horizontal nystagmus, and weakness in the upper and lower limbs. No alterations were found upon physical examination of the lungs, heart, and abdomen. On the right shoulder there was a painless, well-defined ulcerated lesion with raised borders and a blood crust (Figure 1), which was sent for biopsy. Laboratory tests showed leukocytosis (13,900 cells/mm3) with neutrophilia (10,700 cells/mm3), elevated transaminases (AST 67 U/L and ALT 180 U/L) and elevated C-reactive protein (27 mg/L for a normal value of up to 10 mg/L) levels. Serologies for HIV and hepatitis B and C were non-reactive.
Figure 1. Skin lesion presented by the patient on admission. On the left, a painless ulcerated nodule with well-defined limits and regular edges, partially covered by a blood crust. On the right, the same lesion after 34 days of treatment with amphotericin B and flucytosine, with sutures due to the biopsy of the lesion.
A second magnetic resonance imaging (MRI) of the skull was performed and showed that there were in fact multiple cystic lesions, mainly in the nucleocapsular region, in the midbrain, and in the cerebellum (Figures 2 and 3), with no signs of cranial hypertension. The first cerebrospinal fluid (CSF) sample collected by lumbar puncture showed high opening pressure (26 cm/H2O), xanthochromic appearance of the fluid, hyperproteinorrhea (95 mg/dL), and pleocytosis (103 cells/mm3) at the expense of lymphocytes (81%), with positive lateral flow test for cryptococcal antigen and visualization of encapsulated yeast in India ink. Treatment with liposomal amphotericin B 3 mg/kg/day combined with flucytosine 100 mg/kg/day was initiated as soon as the CSF results became available. A few days later, the result of the histological analysis of the biopsied skin was released, showing intense histiocytic inflammation associated with numerous yeasts (Figure 4). Both the CSF and the sample of the skin lesion were sown in culture medium specific for fungi and growth of C. gattii was observed. A third focus of infection was observed on chest computed tomography (CT), where a heterogeneous mass with a diameter greater than 8 cm was found in the middle lobe of the lung (Figure 5).
Figure 2. T1-weighted magnetic resonance imaging (MRI) image of the skull performed on admission, showing cystic lesions initially mistaken for neurocysticercosis.
Figure 3. T2-weighted image of the same skull MRI performed on admission, showing multiple and diffusely distributed cystic lesions, predominantly in the nucleocapsular region, midbrain, and cerebellum. The lesions varied in size, with no associated edema nor gadolinium impregnation.
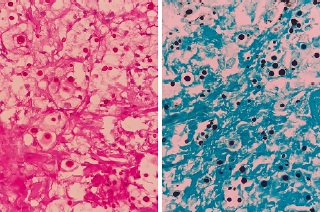
Figure 4. Histological sections of the skin biopsy sample, showing histiocytic inflammation and numerous yeasts compatible with Cryptococcus spp. On the left, periodic acid-Schiff staining; on the right, the Grocott method.
Figure 5. Computed tomography of the chest (left: axial section and right: coronal section), showing a heterogeneous mass in the middle lobe, measuring 69 × 54 × 83 mm3.
Despite the treatment, the patient developed progressive signs and symptoms of intracranial hypertension, confirmed by fundoscopy that showed papilledema. A lumboperitoneal shunt was therefore indicated and performed on the 23rd day of hospitalization. Despite the negative results of the subsequent CSF cultures, the patient continued to suffer from headaches and disorientation; thus, considering a possible case of post-infectious systemic inflammatory syndrome (PIIRS), corticosteroid therapy was initiated (1 mg/kg/day of prednisone for a week, followed by slow weaning). This hypothesis also contributed to the decision to surgically resect the lung mass in order to control the fungal focus, so a middle lobectomy was performed on the 50th day of hospitalization. The operation was uneventful and the anatomopathological study of the surgical specimen and its culture also confirmed Cryptococcus spp. infection. Thenceforth, there was gradual clinical improvement and a reduction in brain lesions on the control images. The patient was discharged on the 86th day of hospitalization, 4 days after initiating the consolidation phase of treatment based on the prescription of fluconazole 900 mg a day.
During outpatient follow-up, the patient presented episodes of neurological worsening that resulted in two readmissions in 2024 to resume the induction therapy regimen with liposomal amphotericin B and flucytosine. On these two occasions, the CSF cultures for fungi remained negative; however, mild hyperproteinorrachia persisted. At the last outpatient visit recorded in the medical records, the patient was clinically stable, asymptomatic, and still taking fluconazole 900 mg a day. A skull MRI performed on the 470th day since the inititiation of cryptococcosis treatment (including three induction regimens alternating with the consolidation phases of high-dose fluconazole) showed the persistence of brain lesions, albeit with significant improvement (Figure 6). The investigation of a possible primary immunodeficiency had shown a slight consumption of C3 (63 mg/dL for a reference value of between 82 and 173 mg/dL), an increase in serum IgE (834 IU/ml for a normal value of less than 100 UI/ml), and no changes in the counts of lymphocyte subpopulations (CD4, CD8, and CD3) in peripheral blood immunophenotyping results. After missing a pre-scheduled outpatient appointment for the second time, an active search for the patient resulted in the news that she had died during an admission to another hospital due to acute pulmonary edema caused by cardiac arrhythmia.
Figure 6. MRI of the skull after 470 days of treatment. There was a reduction in the foci with signal alteration in the T2/FLAIR-weighted sequences. However, sparse nodular lesions can still be seen in both cerebral hemispheres.
DISCUSSION
The initial suspicion of neurocysticercosis in this case was raised because this is the most common parasitic infection of the central nervous system, caused by the ingestion of eggs of Taenia solium4. The diagnosis of neurocysticercosis is primarily based on imaging and the characteristic findings are cystic forms, which can be confused with the mucinous pseudocysts of cryptococcosis (Figure 2). Similarly, chronic forms of neurocysticercosis can also be confused with granulomatous diseases such as tuberculosis and fungal meningitis4-6. The absence of comorbidities or of any type of immunosuppression was also probably taken into account in prioritizing neurocysticercosis as the initial diagnostic hypothesis.
C. neoformans is commonly associated with bird excreta (especially pigeons), which explains its dispersion in more densely populated urban areas. It is considered an opportunistic disease, as it mainly affects HIV-infected patients7,8. Studies on the ecological niches of C. gattii have shown its predilection for plants and wood waste in general, and it is classically related to infections in immunocompetent individuals7. Human cryptococcosis, however, is not a notifiable disease in Brazil.
The molecular pattern of 443 Brazilian isolates of Cryptococcus ssp. (clinical and environmental samples) was analyzed to determine their geographical distribution and the respective risk factors of the hosts9. The genotypes of the C. neoformans complex accounted for 73% of the cases, whereas a regional pattern of cryptococcosis in the North and Northeast regions was observed due to a specific molecular type of C. gatti19. Areas where this endemic pressure exists may reflect a significantly greater association of this agent with AIDS8. Moreover, the genetic diversity of each species complex itself results in different degrees of virulence and pathophysiological characteristics; thus, some variants of C. gattii infect mostly immunocompromised individuals9 and C. neoformans can cause a fatal infection in apparently normal individuals7,8. This may have therapeutic implications, in the sense that not only the medication to be used should be considered, but also the genotypes involved9.
Among the patients with cryptococcosis caused by C. gattii reported in the 1999 outbreak in British Columbia, 38.7% had a known history of immunodeficiency10. However, only 19% had no predisposing factors identified in another outbreak in the Pacific Northwest region of North America11, compared to 72% among 86 patients with C. gattii in Australia12. It appears that patients with this infection present antibodies that inhibit the granulocyte-macrophage colony-stimulating factor, which plays a role in macrophage differentiation in the alveoli, thus resulting in the impairment of the cellular immune response in the lungs13. In a recent study, the presence of this antibody was observed in 76% of patients affected by C. gattii without known underlying immunosuppression, and this may be a hidden risk factor in previously healthy patients14. Moreover, C. gattii has a larger cell diameter and capsule thickness than C. neoformans, which could be related to greater resistance to the host’s immune system and, consequently, a greater capacity for the fungus to remain latent in the body15.
A common challenge in cryptococcosis, which our case also presented, is the presence of multiple foci of fungal infection that are difficult to eradicate, especially the extensive pulmonary involvement concomitant with cryptococcal meningitis, which is a marker of worse prognosis and an unfavorable outcome than isolated pulmonary or meningeal involvement8,16. Surgical resection in this context is a crucial alternative to consider in addition to antifungal therapy, depending on the patient’s clinical condition and the topography of the surgically accessible lesions8,17.
The worsening of the patient’s neurological symptoms after initiating antifungal treatment, despite the negative culture results was attributed to PIIRS. One of the causes of this syndrome may be the unbalanced pro-inflammatory response that results from the release of fungal antigens during treatment, with the immune response itself causing tissue damage18. There is still no consensus on the ideal management of PIIRS; however, evidence suggests that the use of high-dose corticosteroids, followed by slow weaning, is associated with a better neurological outcome18,19 - we believe that this was one of the strategic factors involved in our patient’s clinical improvement and discharge.
There are no official recommendations for a differentiated pharmacological treatment of cryptococcosis caused by C. neoformans or by C. gattii 8,20,21. Management will depend on the assessment of each particular case and the complications related to the condition. However, the guidelines published in 2010 by the Infectious Diseases Association of America (IDSA)20 reinforce the importance of imaging investigations to identify other foci, as well as a more frequent follow-up of patients with C. gattii due to the slower response to treatment than patients with C. neoformans. More recently, the guidelines published in 2024 by international mycology organizations21 recommend that the induction phase be extended (4 to 6 weeks) in non-HIV infected patients with cryptococcal meningitis.
Conclusion
The case presented herein confirms the challenges encountered in the diagnosis and management of cryptococcosis caused by Cryptococcus gattii, especially in patients without apparent immunosuppression. Therapeutic measures included the prolonged use of antifungal drugs, corticosteroids, surgical interventions, and strict clinical monitoring, which enabled the patient’s initial clinical improvement, albeit definitive cure was not achieved. “This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Dao A, Kim HY, Garnham K, Kidd S, Sati H, Perfect J, et al. Cryptococcosis-a systematic review to inform the World Health Organization Fungal Priority Pathogens List. Med Mycol. 2024 Jun 27;62(6):myae043. DOI: 10.1093/mmy/myae043
2. Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. The Case for Adopting the "Species Complex" Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere. 2017 Jan 11;2(1):e00357-16. DOI: 10.1128/msphere.00357-16
3. Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans Clin Infect Dis. 1995 Jul;21(1):28-34. DOI: 10.1093/clinids/21.1.28
4. Hamamoto Filho PT, Rodríguez-Rivas R, Fleury A. Neurocysticercosis: A Review into Treatment Options, Indications, and Their Efficacy. Res Rep Trop Med. 2022 Dec;13:67-79. DOI: 10.2147/RRTM.S375650
5. Kimura-Hayama ET, Higuera JA, Corona-Cedillo R, Chávez-Macías L, Perochena A, Quiroz-Rojas LY, et al. Neurocysticercosis: radiologic-pathologic correlation. Radiographics. 2010 Oct;30(6):1705-19. DOI: 10.1148/rg.306105522
6. Rosa-Júnior M, Cots E, Biasutti C. Teaching NeuroImage: Cryptococcosis in the Central Nervous System Mimicking Neurocysticercosis. Neurology. 2022 Mar 22;98(12):e1302-e3. DOI: 10.1212/WNL.0000000000200053
7. do Carmo FN, de Camargo Fenley J, Garcia MT, Rossoni RD, Junqueira JC, de Barros PP, et al. Cryptococcus spp. and Cryptococcosis: focusing on the infection in Brazil. Braz J Microbiol. 2022;53(3):1321-37. DOI: 10.1007/s42770-022-00744-y
8. Moretti ML, Resende MR, Lazéra MS, Colombo AL, Shikanai-Yasuda MA. Guidelines in cryptococcosis--2008. Rev Soc Bras Med Trop. 2008 Sep-Oct;41(5):524-44. DOI: 10.1590/S0037-86822008000500022
9. Trilles L, Lazéra Mdos S, Wanke B, Oliveira RV, Barbosa GG, Nishikawa MM, et al. Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcus gattii in Brazil. Mem Inst Oswaldo Cruz. 2008 Aug;103(5):455-62. DOI: 10.1590/s0074-02762008000500008
10. Bartlett KH, Cheng PY, Duncan C, Galanis E, Hoang L, Kidd S, et al. A decade of experience: Cryptococcus gattii in British Columbia. Mycopathologia. 2012 Jun;173(5-6):311-9. DOI: 10.1007/s11046-011-9475-x
11. DeBess E, Cieslak PR, Marsden-Haug N, Goldoft M, Wohrle R, Free C, et al. Emergence of Cryptococcus gattii-- Pacific Northwest, 2004-2010. MMWR 2010;59(28):865-8. Disponível em: https://www.cdc.gov/mmwr/pdf/wk/mm5928.pdf
12. Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, et al; Australia and New Zealand Mycoses Interest Group (ANZMIG)-Cryptococcus Study. Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis. 2012 Sep;55(6):789-98. DOI: 10.1093/cid/cis529
13. Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014 Mar 18;5(2):e00912-14. DOI: 10.1128/mBio.00912-14
14. Yang DH, England MR, Salvator H, Anjum S, Park YD, Marr KA, et al. Cryptococcus gattii Species Complex as an Opportunistic Pathogen: Underlying Medical Conditions Associated with the Infection. mBio. 2021 Oct 26;12(5):e0270821. DOI: 10.1128/mBio.02708-21
15. Saidykhan L, Onyishi CU, May RC. The Cryptococcus gattii species complex: unique pathogenic yeasts with understudied virulence mechanisms. PLoS Negl Trop Dis. 2022 Dec 15;16(12):e0010916. DOI: 10.1371/journal.pntd.0010916
16. Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med Mycol. 2019 Feb 1;57(2):133-50. DOI: 10.1093/mmy/myy086
17. Howard-Jones AR, Sparks R, Pham D, Halliday C, Beardsley J, Chen SC. Pulmonary Cryptococcosis. J Fungi (Basel). 2022 Oct 31;8(11):1156. DOI: 10.3390/jof8111156
18. Anjum S, Dean O, Kosa P, Magone MT, King KA, Fitzgibbon E, et al. Outcomes in Previously Healthy Cryptococcal Meningoencephalitis Patients Treated With Pulse Taper Corticosteroids for Post-infectious Inflammatory Syndrome. Clin Infect Dis. 2021 Nov 2;73(9):e2789-e98. DOI: 10.1093/cid/ciaa1901
19. Liu J, Li M, Gan ZQ, Wang YJ, Lin CR, Chen ZL, et al. Postinfectious inflammatory response syndrome in HIV-uninfected and nontransplant men after cryptococcal meningitis. Future Microbiol. 2020 May;15:613-21. DOI: 10.2217/fmb-2019-0252
20. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010 Feb 1;50(3):291-322. DOI: 10.1086/649858
21. Chang CC, Harrison TS, Bicanic TA, Chayakulkeeree M, Sorrell TC, Warris A, et al. Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect Dis. 2024 Aug;24(8):e495-e512. DOI: 10.1016/S1473-3099(23)00731-4
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















