Pre-XDR Tuberculosis Acquired by Probable Occupational Transmission
DOI:
https://doi.org/10.5935/2764-734X.e20251170Keywords:
Tuberculosis, Multidrug-Resistant, Occupational Diseases, Bronchoscopy, Tumor Necrosis Factor Inhibitors, Case ReportAbstract
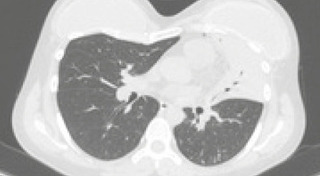
Occupational transmission of tuberculosis is a significant part of its importance to public health, including the possibility of healthcare professionals acquiring resistant forms of the disease in situations of high exposure, such as aerosol-generating procedures. This report describes the case of a healthcare professional who developed primary pulmonary tuberculosis with an extensive pre-resistance profile. The diagnosis was confirmed by molecular and phenotypic tests, and treatment involved bedaquiline, linezolid, clofazimine, and terizidone for a total period of 18 months, with good clinical response. This case highlights the need for precise identification of potential resistance levels in tuberculosis and underscores the importance of administrative, environmental, and individual measures to reduce occupational risks.
Downloads
References
World Health Organization. Global tuberculosis report 2024 [Internet]. 1st ed. Geneva: WHO; 2024 [cited 5 Oct 2025]. Available from: https://www.who.int/publications/i/item/9789240095764
Chen K, Bai C. Occupational adverse effects and protective factors in bronchoscopy. J Thorac Dis. 2019; 11(4):1651–61. doi: 10.21037/jtd.2019.03.73
Associação Brasileira de Normas Técnicas (ABNT). NBR 7256:2022 – Tratamento de ar em estabelecimentos assistenciais de saúde. Rio de Janeiro: ABNT; 2022. [cited 5 Oct 2025]. Available from: https://pt.scribd.com/document/713908019/ABNT-NBR-7256-2022
Brasil. Ministério da Saúde. Manual de recomendações para o controle da tuberculose no Brasil [Internet]. 2nd ed. Brasília: Ministério da Saúde; 2019 [cited 5 Oct 2025]. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil_2_ed.pdf
Da Silva EH, Lima E, Dos Santos TR, Padoveze MC. Prevalence and incidence of tuberculosis in health workers: a systematic review of the literature. Am J Infect Control 2022; 50(7):820–7. doi: 10.1016/j.ajic.2022.01.021
Prado TND, Riley LW, Sanchez M, Fregona G, Nóbrega RLP, Possuelo LG, et al. Prevalence and risk factors for latent tuberculosis infection among primary health care workers in Brazil. Cad Saude Publica 2017; 33(12):e00154916. doi: 10.1590/0102-311X00154916
Franco C, Zanetta DMT. Assessing occupational exposure as risk for tuberculous infection at a teaching hospital in São Paulo, Brazil. Int J Tuberc Lung Dis 2006; 10(4):384-9.
Brasil. Ministério da Saúde. Controle de infecção por Mycobacterium tuberculosis em ambientes de saúde [Internet]. Brasília (DF): Ministério da Saúde; 2023 [cited 5 Oct 2025]. Available from: https://www.gov.br/aids/pt-br/central-de-conteudo/publicacoes/2023/controle-de-infeccao-por-m-tuberculosis-em-ambientes-de-saude_-eletronico_leve.pdf
Brasil. Ministério da Saúde. Informative Note 4/2024-CGTM/DATHI/SVSA/MS: Recomendações técnicas aos enfermeiros para ILTB [Internet]. Brasília (DF): Ministério da Saúde; 2024 [cited 5 Oct 2025]. Available from: https://www.gov.br/aids/pt-br/central-de-conteudo/notas-informativas/2024/nota-informativa-no-42024-cgtm-dathisvsa.pdf
Kedia S, Mouli VP, Kamat N, Sankar J, Ananthakrishnan A, Makharia G, et al. Risk of tuberculosis in patients with inflammatory bowel disease on infliximab or adalimumab is dependent on the local disease burden: a systematic review and meta-analysis. Am J Gastroenterol 2020; 115(3):340-9. doi: 10.14309/ajg.0000000000000527
Brasil. Ministério do Trabalho e Emprego. Ordinance MTP n° 4.219/2022 [Internet]. Brasília (DF): Ministério do Trabalho e da Previdência; 2022 [cited 31 Oct 2025]. Available from: https://www.gov.br/trabalho-e-emprego/pt-br/acesso-a-informacao/participacao-social/conselhos-e-orgaos-colegiados/comissao-tripartite-partitaria-permanente/normas-regulamentadora/normas-regulamentadoras-vigentes/nr-32-atualizada-2023-1.pdf
Brasil. Ministério da Saúde. Informative Note 1/2025 – Atualização das recomendações para tratamento da tuberculose drogarresistente com disponibilização da pretomanida [Internet]. Brasília (DF): Ministério da Saúde; 2025 [cited 5 Oct 2025]. Available from: https://www.gov.br/aids/pt-br/central-de-conteudo/notas-informativas/2025/nota-informativa-no1-2025-cgtm-dathi-svsa-ms.pdf/view
Brasil. Ministério da Saúde. Epidemiological Bulletin of Tuberculosis 2025 [Internet]. Brasília (DF): Ministério da Saúde; 2025 [cited 4 Oct 2025]. Available from: https://www.gov.br/aids/pt-br/central-de-conteudo/boletins-epidemiologicos/2025/boletim-epidemiologico-tuberculose-2025/view
Ministério da Saúde (BR). Manual de diagnóstico laboratorial de tuberculose e micobactérias não tuberculosas no Brasil [Internet]. Brasília (DF): Ministério da Saúde; 2022 [cited 5 Oct 2025]. Available from: https://www.gov.br/aids/pt-br/central-de-conteudo/publicacoes/2022/manual-diagnostico-laboratorial-de-tb-e-micobacterias-nao-tuberculosas-no-brasil_22.pdf/view
Brasil. Ministério da Saúde. Technical Note 7/2024-CGDR/DATHI/SVSA/MS: Orientações para utilização do teste molecular LPA (Line Probe Assay) no diagnóstico da tuberculose drogarresistente [Internet]. Brasília (DF): Ministério da Saúde; 2024 [cited 5 Oct 2025]. Available from: https://www.gov.br/aids/pt-br/central-de-conteudo/notas-informativas/2024/nota-tecnica-no-7-2024-cgdr-dathi-svsa-ms-lpa.pdf
Bhering M, Kritski A. Strengthening multidrug-resistant tuberculosis epidemiological surveillance in Rio de Janeiro: a multidimensional analysis. Rev Soc Bras Med Trop. 2024; 57:e00202-2024. doi: 10.1590/0037-8682-0202-2024
Brasil. Ministério da Saúde. Informative Note 9/2021 – CGDR/DCCI/SVS/MS [Internet]. Brasília (DF): Ministério da Saúde; 2021 [cited 17 Aug 2025]. Available from: https://www.gov.br/aids/pt-br/central-de-conteudo/notas-informativas/2021/nota-informativa-no-9-2021-cgdr-dcci-svs-ms/view
Saukkonen JJ, Duarte R, Munsiff SS, Winston CA, Mammen MJ, Abubakar I, et al. Updates on the treatment of drug-susceptible and drug-resistant tuberculosis: an official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2025; 211(1):15–33. doi: 10.1164/rccm.202410-2096ST
United States of America. CDC Centers for Disease Control and Prevention. Updated Guidelines on the Treatment of Drug-Susceptible and Drug-Resistant TB [Internet]. CDC; 2025 [cited 31 Oct 2025]. Available from: https://www.cdc.gov/tb/php/dear-colleague-letters/2025-treatment-guidelines.html
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2026 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















