Purpura fulminans on invasive meningococcal disease
DOI:
https://doi.org/10.5935/2764-734X.e202201006Keywords:
Meningococcal Infections, Neisseria meningitidis, Serogroup W-135, Purpura FulminansAbstract
Meningococcal disease has been responsible for millions of deaths in its epidemics worldwide. This report aims to highlight the rapid evolution of this currently less prevalent disease and its main complication, purpura fulminans, a rare and highly lethal syndrome, as well as to comment on its clinical and surgical management. This is a 23-year-old female healthcare professional who was diagnosed with meningoccemia caused by serogroup-W N. meningitidis, clinically progressing with purpura fulminans and subsequent need for amputation of the affected segments, in addition to other complications. The patient was not vaccinated for this serogroup, which would have avoided this serious condition - vaccination should be considered for the risk group to which she belonged. It’s an important case report in order to not forget diseases that are allegedly under control, but decades or centuries ago generated major health problems.
Downloads
INTRODUCTION
Meningococcal infection is an epidemic disease responsible for innumerable deaths worldwide, most notably in Sub-Saharan Africa and Southeast Asia. Brazil experienced the largest meningococcal infection epidemic in 1974. Mass vaccination against meningococcus has reduced the incidence of this disease from 179 cases/100,000 inhabitants in 1974 to 1 case/100 thousand inhabitants in 2013 in the country1,2. The incidence of this disease further dropped down to 0.4 cases/100 thousand inhabitants between 2017 and 20203.
This case report aims to highlight the rapid progression of this disease and its main complication, purpura fulminans, a rare and lethal syndrome, along with ofering commentary on its clinical and surgical management. The data in this report were extracted from physical and electronic medical records.
CASE REPORT
The patient was a 23-year-old woman, undergraduate student in Dentistry with a history of otitis media and recurrent pharyngotonsillitis; however, it must be noted that she had had no episodes of infection in the past six months or any other known comorbidity. She had taken all the vaccination doses under the National Immunization Program, except for the meningococcal C and annual influenza vaccines.
She presented with fever, which was sudden in onset, malaise, difuse myalgia, vomiting, and diarrhea. Her condition rapidly progressed to syncope, only 16 h after the onset of initial symptoms. She sought medical care at a medical center close to her place of residence, where she was admitted to the emergency room due to fuctuations in her vital signs (a blood pressure of 83×34 mmHg, a heart rate of 140 bpm, a respiratory rate of 30 bpm, a peripheral oxygen saturation of 70%, and an axillary temperature of 34.3ºC). She was drowsy and had cold, poorly perfused extremities. On physical examination, it was noted that she had no signs of meningeal irritation.
Intravenous infusion of ceftriaxone and metronidazole along with volume expansion using crystalloids was initiated. However, there were no improvements in the clinical parameters of the patient; her condition exacerbated into septic shock, and she was transferred to the intensive care unit (ICU), where she underwent orotracheal intubation and vasoactive drug administration. Although the patient’s condition stabilized eventually, she developed peripheral cyanosis over the next few hours -bilaterally in her ankles and hands and then in the cubital fossa approximating the venous access sites.
Her laboratory tests revealed (Table 1) initial pancytopenia, with subsequent anemia and thrombocytopenia. Owing to her poor blood count, a lumbar puncture for collecting cerebrospinal fluid (CSF) could not be performed on admission. She presented with marked leukocytosis from the second day of admission. In addition, her rapid tests for dengue and human immunodeficiency viruses were non-reactive, with a non-reactive venereal disease research laboratory test and negative serum beta-human chorionic gonadotropin.
| Day of hospitalization | Day 1 | Day 2 | Day 4 | Day 5 | Day 6 |
|---|---|---|---|---|---|
| Hemoglobin | 7,2 | 12,2 | 11,0 | 10,2 | 9,3 |
| Hematocrit | 21,6% | 35,3% | 31,9% | 30,2% | 27,8% |
| Leukocites | 3.200 | 64.300 | 57.300 | 46.800 | 40.500 |
| Neutrophils | 68% | 93% | 78% | 72% | 79% |
| Platelets | 37.000 | 43.000 | 44.000 | 18.000 | 44.000 |
| Urea | 29 | 97 | 97 | 125 | 124 |
| Creatinine | 1,0 | 0,2 | 0,6 | 2,1 | 1,78 |
| Sodium | 137 | 146 | 149 | 156 | 152 |
| Potassium | 3,4 | 3,6 | 3,0 | 2,9 | 3,1 |
| Magnesium | 1,2 | NA | 2,0 | 2,4 | 2,4 |
| arterial ph | NA | 7,33 | 7,48 | 7,53 | 7,53 |
| arterial pO2 | NA | 90 | 85 | 101 | 74 |
| arterial pCO2 | NA | 45 | 31 | 29 | 30 |
| arterial HCO3 | NA | 23 | 23 | 27 | 27 |
| arterial BE | NA | NA | NA | 2 | NA |
| arterial satO2 | NA | 97% | 97% | 98% | 96% |
| arterial lactate | NA | 3,2 | NA | 16 | 16 |
| INR | NA | 1,40 | 1,12 | 1,27 | 1,25 |
| R | NA | NA | 1,0 | 0,9 | 0,9 |
| CRP (mg/dL) | 7,5 | NA | 5,1 | 24,5 | 18,0 |
| AST | 57 | 185 | NA | 69 | 62 |
| ALT | 46 | 95 | NA | 61 | 41 |
| TB | 1,46 | NA | NA | NA | NA |
| IB | 0,80 | NA | NA | NA | NA |
| DB | 0,66 | NA | NA | NA | NA |
| glucose | NA | NA | NA | 235 | NA |
| LDH | NA | NA | NA | 1.336 | 1.086 |
| CPK | NA | NA | NA | 1.712 | 1.690 |
| HIV sorology | - | - | - | NR | - |
| VDRL | - | - | - | NR | - |
| Rapid test for dengue | NR | - | - | - | - |
| Beta HCG test | negativo |
Her blood culture reports, which were available three days post-admission, were positive for Neisseria meningitidis. Serogroup identification of the strain revealed the following results: N. meningitidis serogroup W.
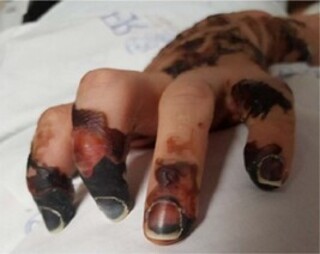
On day five of her illness, the patient was transferred to the ICU of a tertiary referral hospital for infectious diseases, where she clinically progressed satisfactorily. The laboratory tests indicated the onset of acute renal failure, with increased cell injury markers (lactic dehydrogenase and creatine phosphokinase), severe hypokalemia, and pronounced leukocytosis accompanied with bicytopenia (anemia and thrombocytopenia). Concurrently, she presented with the following skin lesions: cyanosis with ill-defined erythematous borders in the distal phalanges of the second and third digits with dry gangrene (Figure 1), hypothermic extremities, purpura with ill-defined erythematous borders interspersed with healthy skin, and blisters with necrosed substance on the wrists, dorsum of the hands, and proximal phalanges (Figure 2). In addition to this, pallor and hypothermia were noted bilaterally on her toes, associated with extensive purpura from the ankles till the proximal phalanges (Figure 3). Her extremities were covered with thermal insulating materials to prevent the progression of devitalization.
Figure 1. Second and third fingers of the left hand (day five of illness)
Figure 2. Right hand (day five of illness)
Figure 3. Left foot (day five of illness)
A computed tomography scan (CT scan) of the brain performed at the tertiary referral hospital showed no changes when the patient was already on day five of antibiotic therapy and had no neurological signs or symptoms. Therefore, lumbar puncture was not performed to collect CSF at this moment.
She was transferred to the ward after 10 days in the ICU. On day 15 post-admission in the ward, the patient showed regression of some purpuric lesions and progression of others; the lesions were localized the tips of her fingers and on both feet. Her feet showed well-defined dry gangrene, and the margins showed erythematous macules interspersed with healthy skin (Figures 4 and 5).
Figure 4. Left foot (day15 of illness)
Figure 5. Left hand (day 15 of illness)
The results of her color Doppler ultrasound ruled out arterial or venous occlusions, indicating possible thrombosis in the limbs. The patient was then evaluated by a surgical team to schedule amputation of the necrotic limbs (feet and distal phalanges of the second and third fingers of the left hand). The team decided to wait for a possible revitalization of the proximal parts of the affected limbs, with better delimitation of necrotic regions, for conservative management of the surgical procedure.
The patient began to have regular episodes of fever in the afternoon, with no clearly defined infectious focus or any other associated symptoms. To investigate the origin of the fever, CT scans of the chest, abdomen, and pelvis were taken. Chest tomography showed moderate pleural efusion in the left hemithorax, while abdominal tomography identified a hypodense image in the topography of the spleen with approximate dimensions of 14 cm×14 cm×5 cm (volume of approximately 1000 mL) and tomographic characteristics of a splenic abscess. Empirical therapy was initiated aiming nosocomial germs with piperacillin + tazobactam and vancomycin. The interventional radiology team performed ultrasound-guided splenic puncture. In addition, 600 mL of liquid with a hematic and purulent appearance was drained, and a drain was placed to monitor the collection.
After local drainage and broad-spectrum antimicrobial coverage, the patient showed resolution of the fever, and disappearance of the fluid collection was observed on control imaging examinations. However, no microorganisms were isolated in the cultures of the drained fluid.
On day 54 post-admission, bilateral transtibial amputation and amputation of the distal phalanges of the second and third left fingers were performed. The surgical sites underwent uneventful healing, and the patient was discharged after a total of 75 days of hospitalization. She was functionally rehabilitated with the placement of orthopedic prostheses in the lower limbs.
DISCUSSION
The patient developed a classic clinical presentation of meningococcemia with rapid and dramatic progression except for the initial presence of diarrhea, a symptom that is uncommon in meningococcal disease4. Since the incidence of meningococcemia has decreased, its initial clinical presentation can confuse the physician in the first visit, leading to a false diagnosis; that is, the patient was admitted with a diagnostic hypothesis of sepsis that progressed to septic shock. In cases of sepsis, aggressive fluid resuscitation and intravenous antibiotic therapy are immediately initiated within the first hour, along with constant clinical reassessment for the need to administer vasoactive drugs to maintain an adequate mean arterial pressure5. This was performed in the case reported here.
The laboratory tests (Table 1) showed some relevant changes, particularly with respect to the initial pancytopenia. Leukopenia denotes extreme severity in an acute infectious condition because leukocytosis is expected as an inflammatory response to a pathogenic agent and can be interpreted as a delay in the initial response to gram-negative bacterial lipopolysaccharides, which are highly inflammatory and may explain the intense leukocytosis that the patient developed in the following days6. Thrombocytopenia is related to the development of coagulopathy related to meningococcemia, which progresses with intravascular coagulation and, classically, depletes procoagulant factors and increases fibrinogen. However, these tests were not initially performed in the case described here. Lactic dehydrogenase and creatine phosphokinase, cell and muscle injury markers, respectively, which were elevated from day five of illness, can be interpreted as evolution of ischemia and tissue necrosis of the extremities. Urea increased from day two of hospitalization onward and creatinine from day five, rapidly progressing to acute renal failure, likely associated with sepsis and, to some degree, with rhabdomyolysis in the extremities. Lastly, the hypernatremia associated with hypokalemia that developed early on during hospitalization may be associated with hyperhydration with unbalanced crystalloid solutions (saline solution) administered during the initial days of hospitalization.
In this case, the patient developed purpura fulminans, a syndrome marked by a rapidly progressive thrombotic injury, which clinically progresses to disseminated intravascular coagulation and multiple organ failure, aggravated by concomitant septic shock5. It is associated with mortality rates ranging from 20 to 60%, in contrast to 15% mortality in uncomplicated meningococcemia7. It begins on the skin as erythematous macules with irregular borders, mainly on the trunk and extremities, which quickly converge and progress to purplish areas with central hemorrhagic necrosis and may progress to blisters with hemorrhagic content. As the disease progresses, necrosis areas become surrounded by erythema which disappears close to healthy skin7,8. Regions with necrosis spanning all the skin layers take four to eight weeks to recover, forming large scars that may require surgical debridement or amputation7, as observed in the case reported here.
Another key point of this case is the fact that a young patient was affected by a disease, which can be prevented by effective vaccination. She was discharged from the hospital with significant morbidities, which further lengthened her recovery period, adversely affecting her quality of life. Coverage by the National Immunization Plan (Plano Nacional de Imunização) of the Unified Health System (Sistema Único de Saúde – SUS) only provides for serogroup C vaccination, which in the case presented here was not the serogroup responsible for the disease. The analysis of this scope highlights the importance of ensuring that all healthcare professionals are properly vaccinated for other serogroups as well because they are more exposed to risk, and vaccination against other serogroups is also available in Brazil. Currently, this condition is not included in the indications for vaccination with the quadrivalent meningococcal conjugate vaccine (MenACWY)9. This vaccination could prevent a future increase in cases of other serogroups that are not part of the National Immunization Program and protect professionals from developing this potentially lethal syndrome that leaves limiting sequelae.
CONCLUSION
The case illustrates the rapid rate of onset of meningococcal disease, as well as its severity and subsequent morbidity, despite initial care without great delay. Purpura fulminans requires debridement of devitalized tissues or timely amputation to preserve as much disease-free area as possible and thus reduce morbidity and rehabilitation time.
Diseases that decades or centuries ago generated major health problems and are currently controlled, either through vaccination or routine prophylactic measures, must not be forgotten because they may arise in everyday life and become a great diagnostic and therapeutic challenge due to their low rates of incidence. We must always be alert and prepared.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access artcle is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Focaccia R. Doença meningocócica. In: Veronesi R, Focaccia R, eds. Tratado de infectologia. 5ª ed. São Paulo: Atheneu; 2015. p. 1053-66.
2. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Situação epidemiológica da doença meningocócica, Brasil, 2007-2013. Boletim epidemiológico - volume 47, nº 29, 2016 [Internet]. Brasília (DF): Ministério da Saúde; 2020; [acesso em 2020 Dez 20]. Disponível em: https://www.gov.br/saude/pt-br/assuntos/boletins-epidemiologicos/numeros-anteriores
3. Ministério da Saúde (BR). Meningite [Internet]. Brasília (DF): Ministério da Saúde; 2020; [acesso em 2020 Dez 20]. Disponível em: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/m/meningite
4. Guiddir T, Gros M, Hong E, Terrade A, Denizon M, Deghmane AE, et al. Unusual initial abdominal presentations of invasive meningococcal disease. Clin Infect Dis. 2018 Oct;67(8):1220-7. DOI: https://doi.org/10.1093/cid/ciy257
5. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov;49(11):e1063-e43. DOI: https://doi.org/10.1097/CCM.0000000000005337
6. Abe R, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Tateishi Y, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care. 2010 Mar;14(2):R27. DOI: https://doi.org/10.1186/cc8898
7. Chalmers E, Cooper P, Forman K, Grimley C, Khair K, Minford A, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96(11):1066-71. DOI: https://doi.org/10.1136/adc.2010.199919
8. Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018 Apr;32(2):69-76. DOI: https://doi.org/10.1016/j.tmrv.2017.10.001
9. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Imunização e Doenças Transmissíveis. Coordenação-Geral do Programa Nacional de Imunizações. Manual dos centros de referência para imunobiológicos especiais [Internet]. 5ª ed. Brasília (DF): Ministério da Saúde; 2019; [acesso em 2020 Dez 20]. Disponível em: https://pesquisa.bvsalud.org/bvsms/resource/pt/mis-40552
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















