Syphilis as the cause of disseminated ulcerated crusted skin lesions in an immunosuppressed patient
DOI:
https://doi.org/10.5935/2764-734X.e202205011Keywords:
Syphilis, Cutaneous, Acquired Immunodeficiency Syndrome, Warts, Case ReportAbstract
Individuals infected with the human immunodeficiency virus (HIV) have a higher risk of co-infection with syphilis, and may have a different course of evolution from those who are seronegative, sometimes faster and more aggressively. The manifestation of secondary syphilis is proportionately more prevalent, with malignant syphilis (MS) being a rare form of this stage of the disease, but with a significant increase in the number of cases after the AIDS epidemic. MS presents with pleomorphic lesions, classically described as disseminated ulcero-nodular lesions, accompanied by more intense constitutional symptoms. This report describes the case of a female patient whose diagnosis of HIV was simultaneously established with that of recent syphilis, which occurred during the investigation of disseminated, ulcerated and crusted skin lesions, sometimes with a rupioid appearance. The diagnosis of secondary syphilis was established in association with asymptomatic neurosyphilis. Despite the clinical and laboratory criteria compatible with the diagnosis of MS, the infrequent cutaneous presentation deserved the differential diagnoses grouped as a verrucous syndrome, especially sporotrichosis.
Downloads
INTRODUCTION
The recent change in terminology from sexually transmitted disease (STD) to sexually transmitted infection (STI) added an important warning about exposure and risk perception to syphilis. It is a chronic infection caused by the spirochete Treponema pallidum, whose clinical manifestations vary according to the stage of the disease and represent a diagnostic challenge. The primary form occurs between two to six weeks after exposure, characterized by the appearance of a hard chancre at the inoculation site – a shallow, clean-bottomed ulcer that heals regardless of treatment. The secondary form is established between one and two months after the primary, and the most characteristic manifestation is generalized skin rash with palmoplantar involvement, which may present with systemic symptoms (fever, lymph node enlargement, hepatitis, nephritis). The early latent phase represents the asymptomatic individual with less than one year of infection and can only be diagnosed through serological tests. In this period, in up to 24% of cases, recurrence of secondary lesions may occur, a risk that reduces after one year of infection, in the late latent phase. From this moment on, about 70% of individuals will remain in latency for life, while another 30% may progress to one of the tertiary forms of the disease (neurosyphilis, cardiovascular, gummatous syphilis), in a period ranging from 2 to 50 years after initial infection1.
Although syphilis is not considered an opportunistic infection, its incidence is increased in the HIV population. Both diseases interfere with each other’s progress. Syphilitic lesions, especially the primary ulcer, lead to the breakdown of the epithelial and mucosal barrier, facilitating the entry of the virus and thus increasing the risk of HIV acquisition in the first six months of exposure to Treponema. The primary chancre is deeper, with a larger diameter and longer time required for healing, and 25% of co-infected patients have concomitant primary and secondary lesions at the time of diagnosis2,3. Co-infection generates a transient increase in viral load and a reduction in the CD4 T-cell count, reversible after syphilis treatment. The clinical presentation is mostly similar to seronegative patients, but with specific characteristics, such as a rapid and sometimes aggressive progression, in addition to a greater predisposition to neurosyphilis.
Malignant syphilis (MS) is included in the secondary stage of the disease, represented by greater atypia and variability of cutaneous lesions. MS is rare, associated with immunosuppression in general (with especially high incidence in HIV-positive individuals), and classically described by multiple pleomorphic, ulcero-nodular lesions with more intense constitutional symptoms4,5. The present report describes a case of a patient who presented MS and neurosyphilis as the initial manifestation of AIDS, with skin lesions that, despite being compatible with the diagnostic criteria of lues maligna, deserved a wide spectrum of differential diagnoses.
CASE REPORT
A 40-year-old female patient was admitted to the emergency department of a tertiary hospital specializing in infectious diseases with a complaint of skin lesions that had appeared three months before. These lesions were initially described as erythematous-violaceous papules in the anterior region of the chest, followed by new lesions of an additive nature, which generalized throughout the body, progressing with ulceration of the papules and formation of coarse crusted plaques. She also reported asthenia and inappetence, with an estimated weight loss of 6 kg. She denied fever, respiratory, neurological, and gastrointestinal changes. No previous comorbidities were reported, and she was not taking any continuous medication, though she had a past history of drug addiction. She had lived in an urban area in the state of São Paulo for over 20 years and had cats as pets.
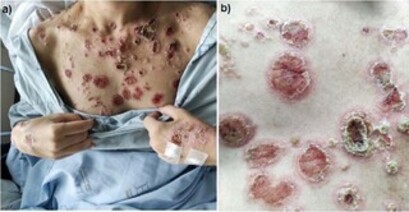
Upon initial examination, she had widespread erythematous scaly macules and patches, interspersed with ulcerated, crusty vegetating plaques, sometimes with a rupioid appearance (in which there are several layers of thick crusts similar to the shell of an oyster covering the ulcerations), more intense in the thoracic and distal extensor aspect of limbs, without mucosal or palmoplantar involvement [Figures 1 and 2].
Figure 1. A. Crusted ulcerated plaques, some with a vegetative or rupioid appearance, disseminated in the thorax region and dorsal surface of the hands; B. Same injuries in closer detail.
Figure 2. Erythematous, scaly papules and plaques in the anterior region of the legs and medial region of the left thigh.
The initial diagnostic hypotheses involved diseases such as verrucous syndrome, and the dermatologist in charge considered sporotrichosis as the main hypothesis, given the characteristics of the lesions and compatible epidemiology.
However, positive serology for HIV was confirmed by immunoblotting in the admission exams, in addition to a positive treponemal test (chemiluminescence) and titration in serum of a non-treponemal Venereal Disease Research Laboratory (VDRL) test of 1:512. The patient denied previous knowledge of these diagnoses. CD4 T-lymphocyte (CD4 TL) count resulted in 249 cells/mm3 and the HIV viral load was 102,152 copies/mL (log). Biopsy of the skin lesion showed a lymphoplasmacytic dermal infltrate with perivascular and periadnexal deposition, extending to the interface with the epidermis, and presence of acantholysis, compatible with chronic dermatitis with an acute component. No granulomatous pattern was observed, and alcohol-acid fast bacillus (AAFB) and fungi were negative by specific staing methods. The immunohistochemical analysis was positive for the antigen of Treponema spp, while negative for herpes simplex virus (HSV).
As an initial approach, therapy was prescribed for secondary syphilis (intramuscular benzathine penicillin G 2,400,000 IU) and presumptive treatment for herpes (intravenous acyclovir 450 mg 8/8h) in view of the polymorphism of the lesions. Antiretroviral therapy with tenofovir (TDF), lamivudine (3TC), and dolutegravir (DTG) was also started after negative screening for tuberculosis (chest tomography without suggestive changes) and cryptococcosis (negative antigen in serum).
Faced with the diagnosis of syphilis in a patient with CD4 TL less than 350 cells/mm3, the patient underwent computed tomography of the skull (no changes), eye fundoscopy (no changes) and cerebrospinal fluid (CSF) collection, whose result confirmed an asymptomatic neurosyphilis: 109 cells/mm3 with a predominance of 93% lymphomonocytes, increased levels of protein concentration in CSF (110 mg/dL; reference value 40mg/dl), and positive immunology (positive FTA-ABS and VDRL positive 1:4). Other screening tests on CSF were negative (Gram stain, AAFB, fungus, and China ink).
Syphilis treatment was then changed to crystalline penicillin G (4,000,000 IU 4/4h, intravenous), while acyclovir was discontinued after skin biopsy results. After nine days of adequate treatment with intravenous penicillin, the patient requested discharge from the hospital, a decision that was not reversed even after recommendations to the contrary by the assistant medical team. The patient was prescribed intravenous ceftriaxone 1g 12/12h, to complete 14 days of treatment on an outpatient basis in her city of origin. However, the patient did not return and there was a loss of follow-up after discharge.
DISCUSSION
Several reports present the concomitant diagnosis of syphilis and HIV infection, similar to this case, including a detailed description of negative serologies documented before the diagnosis of co-infection6. People living with HIV (PLHIV) are eight times more likely to acquire syphilis compared to the general population2. In these individuals, the spectrum of clinical presentation is wide, especially in the secondary form of the disease, represented by atypical cutaneous findings, with faster progression and greater severity of lesions, in addition to an earlier progression to tertiary stages and a greater probability of disease relapse, even after adequate treatment with penicillin7.
MS is one of these atypical forms. Initially described as a tertiary presentation of the disease by Bazin in 1859, it was later reclassified as an aggressive variation of the secondary in 18964. The key features that differentiate it from a late form include the presence of multiple pleomorphic papules, with ulcero-nodular, ovoid, and crusted lesions, with a short incubation period and a four-week prodrome before the skin eruptions8.
Before the HIV/AIDS epidemic, only 14 cases had been reported in the literature, and MS was a clinical entity considered to be extremely rare9. The first report in an HIV-positive patient was described in 1987, followed by a growing increase in subsequent years to the point of reaching an estimated incidence 60 times higher than the general population10,11. In a recent systematic review, 45 cases of MS reported between 2014 and 2018 were analyzed; 73.3% of the patients were HIV positive and, among these, more than half had a CD4 TL count between 200-499 cells/mm3 – a fact demonstrating that MS is not necessarily associated with severe immunosuppression, as in the case of the patient reported here5.
The classic criteria considered for the diagnosis of MS (described by Fisher et al.) are high titers in a non-treponemal test, severe Jarisch-Herxheimer (JHR) reaction, compatible macroscopic and microscopic characteristics, and rapid resolution of the lesions after the institution of adequate treatment6. In the case described here, signs were compatible with the diagnosis of MS, such as high serum VDRL titers, rapid progression with ulceration of the lesions and perivascular dermal involvement. However, the patient did not progress with JHR (which was also observed in most of the cases described more recently5), and it was not possible to characterize the rapid clinical improvement with the treatment due to post-discharge loss of follow-up.
Regarding the appearance of the lesions, the most common cutaneous findings of MS are ulcerated nodular lesions, different from that presented by the patient5. For this reason, the initial diagnostic hypotheses were diseases with the presentation of verrucous syndrome, which includes infections with cutaneous manifestations of crusted, vegetative, or verrucous ulcers. These diseases are recognized in dermatology by the acronym PLSCT, namely paracoccidioidomycosis, cutaneous leishmaniasis, sporotrichosis, chromomycosis, and cutaneous tuberculosis12.
Regarding neurosyphilis, its higher frequency in PLHIV makes the indications for lumbar puncture less restrictive. Studies show that cerebrospinal fluid alterations (pleocytosis and increased levels of protein concentration in CSF) are more common in individuals with an CD4 TL count < 350 cells/mm3 and a serum VDRL titer ≥ 1:32213. The latter was considered by Libois et al. as the best predictor of lumbar puncture indication in asymptomatic patients, with a sensitivity of 100%14. These two markers were present in the case described, and the diagnosis of neurosyphilis was confirmed by the positivity of CSF in the VDRL test, a more specific method, but with low sensitivity2.
The most recent Brazilian guidelines do not consider the CD4 TL count or serological titrations as criteria to indicate cerebrospinal fluid puncture, which is only indicated in symptomatic patients (neurological or ophthalmological) or who have evidence of active tertiary syphilis, as well as in cases of therapeutic failure15. If the management of this patient had been limited to these criteria, there would have been no indication for performing lumbar puncture and the diagnosis of asymptomatic neurosyphilis would not have been made, nor would the therapeutic regimen be changed.
CONCLUSION
In this report, syphilis - the “great imitator”, as referred by Sir William Osler - assumes a rare and atypical form, with lesions in papules and ulcerated and crusted plaques, easily confused with other disseminated infections – which in fact occurred, particularly because the patient had a positive epidemiological history of mycoses endemic in Brazil. In this context, we suggest the inclusion of syphilis among the differential diagnoses for verrucous syndrome in HIV-positive patients.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access artcle is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Ghanem KG, Ram S, Rice PA. The Modern Epidemic of Syphilis. N Engl J Med. 2020; 382(9):845-854.
2. Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Eur J Intern Med. 2009; 20(1):9-13.
3. Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007; 44(9):1222-8.
4. Cid PM, Cudós ES, Zamora Vargas FX, Merino MJ, Pinto PH. Pathologically confirmed malignant syphilis using immunohistochemical staining: report of 3 cases and review of the literature. Sex Transm Dis. 2014; 41(2):94-7.
5. Wibisono O, Idrus I, Djawad K. Sífilis maligna: revisión sistemática de los casos publicados entre los anos 2014-2018. Actas Dermosifiliogr. 2021; 112:725-734.
6. Fisher DA, Chang LW, Tuffanelli DL. Lues maligna: presentation of a case and a review of the literature. Arch Dermatol. 1969; 99(1):70-3.
7. Gregory N, Sanchez M, Buchness MR. The spectrum of syphilis in patients with human immunodeficiency virus infection. J Am Acad Dermatol. 1990; 22:1061-7.
8. Sands M, Markus A. Lues maligna, or ulceronodular syphilis, in a man infected with human immunodeficiency virus: case report and review. Clin Infect Dis. 1995; 20:387-90.
9. Costa PP, Moura AA, Rodrigues FLM, Almeida MP, Vilasboas V, Francesconi F. Sífilis maligna precoce em paciente imunodeprimido. Revista SPDV 2019; 77(2).
10. Rosenheim M, Brucker G, Leibowitch M, Niel G, Bournerias I, Duflo B, et al. Syphilis maligne chez un malade porteur d’anticorps anti-HIV [FRE]. Presse Med. 1987; 16(16):777.
11. Santos TR, Castro IJ, Dahia MM, Azevedo MC, Silva GA, Motta RN, et al. Malignant syphilis in an AIDS patient. Infection. 2015; 43(2):231-6.
12. Costa F, Martinez C, Azulay L. PLECT: enfermedades tropicales de manifestación verrucosa. Rev chil dermatol. 2018; 34(3):89-94.
13. Marra CM, Maxwell CL, Smith SL, Lukehart SA, Rompalo AM, Eaton M,et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004; 189:369-76.
14. Libois A, De Wit S, Poll B, Garcia F, Florence E, Del Rio A, et al. HIV and syphilis: when to perform a lumbar puncture. Sex Transm Dis. 2007; 34(3):141-4.
15. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo HIV em adultos. Brasília: MS; 2018.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















