Pericarditis due to Mycobacterium kansasii in a person living with HIV
DOI:
https://doi.org/10.5935/2764-734X.e202205012Keywords:
Pericarditis, Mycobacterium kansasii, HIV, Case ReportAbstract
With regard to ethology, acute pericarditis due Mycobacterium tuberculosis is one of the most prevalent in our country in the context of AIDS. In this paper, we report the first Brazilian case with a surprising diagnosis of pericarditis by M. kansasii due to the late culture survey, highlighting its clinical and laboratory characteristics (presumably compatible with tuberculosis), as well as the therapeutic implications of this result.
Downloads
INTRODUCTION
The heart is an organ commonly affected by ischemic, opportunistic and neoplastic damage and by drug toxicity related to the progression of human immunodeficiency virus (HIV) infection1. Pericardial involvement secondary to opportunistic diseases was frequent in people living with HIV-acquired immunodeficiency syndrome (AIDS) in the era before antiretroviral therapy (ART). In the post-ART era, arrhythmia, acute coronary syndrome, and chronic arterial disease are the main cardiovascular diseases affecting these patients, with a reduced prevalence of pericarditis2.
Acute pericarditis is an inflammation of the pericardial membranes resulting from a variety of stimuli that trigger a stereotyped immune response3 whose clinical presentation ranges from chest pain to classic symptoms of cardiac tamponade4.
As for the etiology, in addition to neoplasms (lymphoma, Kaposi’s sarcoma), there is a broad spectrum of microorganisms already reported in acute pericarditis related to AIDS, including pyogenic bacteria, mycobacteria, viruses, and fungi (Cryptococcus neoformans, Nocardia asteroides, Aspergillus)5,6, with tuberculosis (TB) being the most frequent and probable, especially in countries with a high prevalence7, as is the case of Brazil.
Pericarditis caused by nontuberculous mycobacteria (NTM) is rare, and Mycobacterium avium is the main etiology in this context6. Pericarditis by Mycobacterium kansasii is even rarer, with only nine cases reported in the literature8, 9, 10, 11, 12, 13, 14. This study is the report of the first case of a Brazilian patient diagnosed with pericarditis caused by M. kansasii.
CASE REPORT
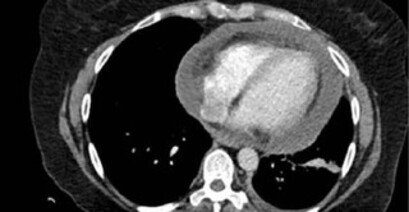
A 48-year-old woman sought emergency care with a complaint of insidious, stabbing retrosternal pain not related to physical exertion, that had been progressing for eight days, and a subfebrile episode (T = 37.7 °C). She had been diagnosed with HIV infection 14 months prior, with a history of Pneumocystis pneumonia and neurocryptococcosis as opportunistic diseases. She had been on regular ART since diagnosis. Upon physical examination, she was observed to be in good general condition, with stable vital signs, without changes on cardiac and pulmonary auscultation. She underwent electrocardiography (ECG), laboratory tests, and chest tomography (Figure 1A), presenting only an increased CK-MB of 70 U/L (RV ≤ 25 U/L), which was considered as abnormal. Having ruled out an ischemic coronary cause, the diagnosis of costochondritis and myocarditis was considered, and she was discharged with a prescription for a non-steroidal anti-inflammatory drug.
Figure 1. A. Non-contrast chest computed tomography (CT) performed during the first appointment (eight days after symptom onset) showing no abnormality in the mediastinal window; B. Contrast chest CT performed during the second appointment (15 days after symptom onset) showing pericardial efusion with a thickness of up to 3.6 cm and mild pleural efusion on the left.
One week later, the patient returned with improved retrosternal pain; however, she reported four days of cough, pain in the left shoulder region, headache, nausea, and a peak fever of 38.0 °C. Upon physical examination at readmission, she presented a transmission snore on pulmonary auscultation and pericardial rub on cardiac auscultation. The patient had a heart rate of 104 bpm and peripheral oxygen saturation of 95%. Laboratory tests showed a non-infectious blood count, no left shift, CRP value of 337.4 mg/L (RV 5–10 mg/L), CK-MB value of 47 U/L, CD4 lymphocyte count of 123 cells/mm3, and an undetectable HIV viral load. Blood samples were collected for aerobic bacteria and mycobacteria culture, which were negative. The ECG remained normal. Cranial computed tomography (CT) showed small bilateral nuclear-capsular hypoattenuating foci and slight global brain volume reduction; additionally, these changes were attributed to previous neurological cryptococcosis (with pseudocysts, already on maintenance therapy with fuconazole) and to the HIV infection itself. Lumbar puncture revealed normal opening pressure and cerebrospinal fluid analysis showed no abnormalities. Chest CT showed moderate pericardial efusion and mild pleural efusion on the left (Figure 1B), with no lung parenchyma changes. Transthoracic echocardiography was not available at the service at that time. Due to cough and fever in the context of the COVID-19 pandemic, the patient underwent a PCR test for SARS-CoV-2, which was negative. The search for acid-fast bacilli (AFB) in the sputum (two samples) and the rapid molecular test for TB (RMT-TB) were negative.
Once the diagnosis of acute pericarditis was established (still without a defined etiology), a treatment consisting of prednisone 40 mg/day and colchicine 0.5 mg every 12 hours were started. Myocardial involvement was not considered in view of the normal troponin level. The patient was stable, with no signs of cardiac tamponade. On the third day of hospitalization, the patient underwent a surgical procedure to create a pleural-pericardial window to drain the efusion and biopsy the pericardium. Approximately 300 ml of cloudy and erythrocytic fluid were drained, and an underwater sealed drain was kept until postoperative day four.
An analysis of the pericardial fluid collected during surgery showed a pH of 7.58; 2,450 cells/mm3, with a predominance of lymphomononuclear cells; protein, 6.4 g/dl; glucose, 51 mg/dl; lactate dehydrogenase (DHL), 3,724 mg/dl; adenosine deaminase (ADA), 83 IU/L; and negative RMT-TB. Direct fungal analysis was negative, as Gram and Ziehl-Neelsen (ZN) stains showed no bacteria or AFB. Subsequently, the samples were sent for culture.
Pericardial biopsy stained with hematoxylin-eosin (H&E) revealed chronic pericarditis with fibrosis, mild granulomatous lesions, and marked fibrin deposition associated with the presence of rare mycobacteria (1+/3+) evidenced by ZN (Figures 2, 3, and 4). Fungal analyses by periodic acid-Schif (PAS) and Grocott stains were negative. A complementary immunohistochemical panel was carried out, and mycobacterium/BCG and cytomegalovirus (CMV) antigen tests were negative.
Figure 2. Photomicrograph of the parietal pericardium segment with chronic inflammatory infltrate and marked fibrosis (H&E - 40X).
Figure 3. Exuberant chronic lympho-histiocytic inflammation with rare granulomatous lesions (between arrows) in the pericardial tissue (H&E - 200 X).
Figure 4. Presence of mycobacteria isolated in histiocytic pericardial inflammation cells (ZN - 1,000X).
Once the diagnostic hypothesis of TB pericarditis was considered, the patient was started with rifampicin (750 mg/day), isoniazid (375 mg/day), pyrazinamide (2,000 mg/day), and ethambutol (1,375 mg/day), according to the patient’s weight (80 kg), in addition to prophylactic pyridoxine associated with isoniazid for peripheral neuropathy. Colchicine was discontinued and prednisone was increased to 80 mg (1 mg/kg/day). An echocardiography performed on postoperative day three showed mild pericardial efusion without signs of restriction or constriction and preserved left ventricular ejection fraction (59%).
After 16 days of hospitalization, still clinically stable, the patient was discharged with a prescription for an anti-TB regimen, pyridoxine, ART (tenofovir 300 mg, lamivudine 300 mg, and dolutegravir 50 mg, 12/12h), prednisone tapering strategy, and appropriate prophylaxis for opportunistic diseases. A follow-up was scheduled at the outpatient service of origin.
Mycobacterium kansasii growth was identified five weeks after the surgical procedure by oligochromatography. A minimal inhibitory concentration test demonstrated resistance to ethambutol and sensitivity to rifampicin, rifabutin, ciprofloxacin, amikacin, and clarithromycin. However, when we contacted the patient to have her informed consent for the publication of this case report (more than 10 months after hospitalization), we found that she was unaware of the culture result, as well as the medical team that followed her on an outpatient basis: the treatment for tuberculosis had been maintained for six months and she had been discharged as cured, with no apparent complications. We communicated this culture result to the medical team about four months after the end of the treatment. The team decided not to initiate specific treatment for M. kansasii, prioritizing clinical surveillance through outpatient follow-ups. So far (16 months after diagnosis), the patient has remained stable, with no evidence of pericarditis recurrence.
DISCUSSION
Nontuberculous mycobacteria (NTM) rarely develop in healthy individuals and were originally considered as saprophytes. It was not until the 1950s that these microorganisms were recognized as human pathogens15. Most diseases caused by NTM in patients living with HIV are caused by the Mycobacterium avium complex, with M. kansasii being the second most frequently isolated NTM in the United States and Japan16. M. kansasii was first described in 195317 and, in contrast to other NTM, it is rarely isolated from natural water sources or from the soil — the main reservoir appears to be tap water. The route of transmission seems to be through aerosols, with no person-to-person transmission18.
Pulmonary disease is the most common form of M. kansasii infection and presents mainly in patients with underlying pulmonary comorbidities. Extrapulmonary disease by M. kansasii is a rare event, and the most common sites include the lymph nodes, skin, and musculoskeletal and genitourinary systems18. In contrast to M. tuberculosis infection, M. kansasii pericarditis has been rarely reported13.
When associated with HIV infection, it usually corresponds to an advanced state of immunosuppression attested by a mean CD4 count of less than 50/mm.3,17 Respiratory and gastrointestinal tract colonization by M. kansasii can be common in these patients; however, the disease can also be disseminated and, more rarely, focal, such as in septic arthritis and pericarditis19.
The majority of the nine cases of pericarditis due to M. kansasii found in the literature were associated with a predisposing condition, such as myelodysplastic syndrome, drug immunosuppression after kidney transplantation, and chronic kidney disease on dialysis; five of these patients had the disease associated with HIV infection8, 9, 10, 11, 12, 13, 14. Of these five, the reports presenting CD4 counts showed values lower than 50/mm3 8,13. The higher CD4 count in our case may be explained by the regular use of ART for at least one year. Even so, her CD4 level still indicates severe immunosuppression.
Clinically, the presentation described was similar to the one of classic acute pericarditis, with febrile syndrome, symptomatic involvement of the upper airways, chest pain, and pericardial rub20. In the five previously reported cases associated with HIV, the clinical condition was also similar, with fever and chest pain as the main symptoms, and pericardial efusion evidenced on imaging tests.
An analysis of the pericardial fluid in the present case revealed that it was an exudate with an increased leukocyte count and predominance of lymphomononuclear cells, a finding compatible with classic TB pericarditis14. However, four of the other published cases of pericarditis due to M. kansasii showed differential cellularity with a predominance of polymorphonuclear cells. Furthermore, the ADA level is notably increased, as reported by Pintado et al.13 and Cho et al.14 As for AFB analysis in the pericardial fluid, five of the nine reported cases were positive, with late M. kansasii growth in culture, such as in our Brazilian case.
Palmer and Watanakunakom reported a case of pericarditis due to M. kansasii in which chronic granulomatous inflammation was described on histology12. In our case, the presumptive diagnosis of TB pericarditis was also supported by histological testing and positive AFB. Negative anti-BCG antibody immunohistochemistry was expected, since mycobacteria have been rarely identified in direct tissue analysis (ZN), emphasizing the low amount of etiologic agent antigen in the analyzed sample. It is also important to infer that the aforementioned anti-BCG mycobacterium antibody has a low specificity, not allowing the differentiation of the mycobacteria groups, TB and non-TB21.
As for the treatment, the patient progressed with a clinical cure and no recurrence despite the inadequate treatment. According to the manual22 recently published by the Brazilian Ministry of Health, which corroborates the guidelines of the American Thoracic Society23, uncomplicated M. kansasii disease should be treated with rifampicin, isoniazid, and ethambutol. This treatment should be modified in case of resistance to rifampicin, in the absence of a susceptibility test, and/or in the severe cavitary form of the disease. There are no individualized treatment recommendations for ethambutol resistance. The suggested treatment time is 12 months after bacteriological conversion or 18 months in case a new culture is not possible. The recent European guidelines for the treatment of NTM lung disease associated current rifampicin-based treatment regimens with a high success rate if used for at least 12 months. It’s to be considered that while randomized clinical trials comparing shorter treatment regimens have been lacking, there is no evidence that relapses can be avoided with treatment courses longer than 12 months24. Importantly, this reasoning has been adopted for lung disease and we do not know whether they can be extrapolated to other sites.
Pintado et al.13 also cited adjuvant corticosteroid therapy in their report. Although this treatment was originally recommended in the context of TB pericarditis, evidence demonstrates a decreased efusion volume and overall mortality probably secondary to efusion control when it is hemodynamically threatening25. However, a recent study26 showed that corticosteroid therapy has no impact on the mortality and constrictive pericarditis rates in people with advanced HIV. The concept of this association should be cautiously extended to patients infected with NTM.
Prior to the introduction of highly active antiretroviral therapy (HAART), M. kansasii infection associated with HIV had a poor prognosis, with a median survival of 12 months27, although most deaths were not directly attributed to M. kansasii infection but to other complications secondary to advanced AIDS. The overall survival of these patients has greatly improved since the introduction of HAART; however, the mortality associated with M. kansasii is still high due to its usual association with advanced immunosuppression18.
CONCLUSION
This case report brings relevant knowledge in the context of AIDS-associated pericarditis considering the unusual diagnosis of M. kansasii. It is essential to confirm Mycobacterium culture results from any biological sample in this group of patients, since the very probable diagnosis of TB (especially in case of pericardial fluid with predominance of lymphomononuclear cells and increased ADA or of granulomatous pericarditis with a positive AFB test) is only presumptive and, if wrong, implies inadequate treatment.
It is also essential to guarantee the effective sharing of information related to late culture results through all available means and channels already existing in the National and State Tuberculosis Control Programs: every professional at diagnostic and care departments is implicitly co-responsible for “always checking culture results” as a clinical practice routine.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access artcle is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Vandi G, Calza L, Girometti N, Manfredi R, Musumeci G, Bon I, et al. Acute onset myopericarditis as unusual presentation of primary HIV infection. Int J STD AIDS. 2017 Fev;28(2):199-201. DOI: https://doi.org/10.1177/0956462416654852
2. Pham TV, Torres M. Human immunodeficiency virus infection-related heart disease. Emerg Med Clin North Am. 2015 Ago;33(3):613-22. DOI: https://doi.org/10.1016/j.emc.2015.04.009
3. Chiabrando JG, Bonaventura A, Vecchié A, Wohlford GF, Mauro AG, Jordan JH, et al. Management of acute and recurrent pericarditis: JACC state-of-the-art review. J Am Coll Cardiol. 2020 Jan;75(1):76-92. DOI: https://doi.org/10.1016/j.jacc.2019.11.021
4. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014 Out;130(18):1601-6. DOI: https://doi.org/10.1161/CIRCULATIONAHA.114.010376
5. Estok L, Wallach F. Cardiac tamponade in a patient with AIDS: a review of pericardial disease in patients with HIV infection. Mt Sinai J Med. 1998 Jan;65(1):33-9.
6. Gowda RM, Khan IA, Mehta NJ, Gowda MR, Sacchi TJ, Vasavada BC. Cardiac tamponade in patients with human immunodeficiency virus disease. Angiology. 2003 Jul/Ago;54(4):469-74. DOI: https://doi.org/10.1177/000331970305400411
7. Canelas C, Carvas JM, Fontoura JI, Lemos J. Pericarditis: not all are idiopathic. Galicia Clin. 2017;78(1):31-4.
8. Moreno F, Sharkey-Mathis PK, Mokulis E, Smith JA. Mycobacterium kansasii pericarditis in patients with AIDS. Clin Infect Dis. 1994 Nov;19(5):967-9.
9. Campo RE, Campo CE. Mycobacterium kansasii disease in patients infected with human immunodeficiency virus. Clin Infect Dis. 1997 Jun;24(6):1233-8.
10. Bacon ME, Whelan TV, Mahoney MD, Patel TG, Judson PL. Pericarditis due to Mycobacterium kansasii in a patient undergoing dialysis for chronic renal failure. J Infect Dis. 1985 Out;152(4):846-7.
11. Natori S, Morimoto H, Matsuzaki M, Matsuhashi H, Onodera S. A case of myelodisplastic syndrome with pericarditis due to atypical Mycobacterium. Nippon Kyobu Shikkan Gakkai Zasshi. 1994;32(1):101-5 apud Pintado V, Fortún J, Casado JL, Gómez-Mampaso E. Mycobacterium kansasii pericarditis as a presentation of AIDS. Infection. 2001 Jan/Fev;29(1):48-50.
12. Palmer JA, Watanakunakom C. Mycobacterium kansasii pericarditis. Thorax. 1984 Nov;39(11):876-7.
13. Pintado V, Fortún J, Casado JL, Gómez-Mampaso E. Mycobacterium kansasii pericarditis as a presentation of AIDS. Infection. 2001 Jan/Feb;29(1):48-50.
14. Cho JH, Yu CH, Jin MK, Kwon O, Hong KD, Choi JY, et al. Mycobacterium kansasii pericarditis in a kidney transplant recipient: a case report and comprehensive review of the literature. Transpl Infect Dis. 2012 Out;14(5):E50-5. DOI: https://doi.org/10.1111/j.1399-3062.2012.00767.x
15. Theodorou DJ, Theodorou SJ, Kakitsubata Y, Sartoris DJ, Resnick D. Imaging characteristics and epidemiologic features of atypical mycobacterial infections involving the musculoskeletal system. Am J Roentgenol. 2001 Fev;176(2):341-9.
16. Han SH, Kim KM, Chin BS, Choi SH, Lee S, Kim MS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010 Jan;25(2):304-8. DOI: https://doi.org/10.3346/jkms.2010.25.2.304
17. Buhler VB, Pollak A. Human infection with atypical acid-fast organisms; report of two cases with pathologic findings. Am J ClinPathol. 1953 Abr;23(4):363-74 apud Johnston JC, Chiang L, Elwood K. 2017. Mycobacterium kansasii. Microbiol Spectr. 2017 Jan;5(1). DOI: https://doi.org/10.1128/microbiolspec.TNMI7-0011-2016
18. Johnston JC, Chiang L, Elwood K. Mycobacterium kansasii. Microbiol Spectr. 2017 Jan;5(1). DOI: https://doi.org/10.1128/microbiolspec.TNMI7-0011-2016
19. Manfredi R, Nanetti A, Valentini R, Ferri M, Morelli S, Calza L. Epidemiological, clinical and therapeutic features of AIDS-related Mycobacterium kansasii infection during the HIV pandemic: an 11-year follow-up study. HIV Med. 2004 Nov;5(6):431-6.
20. Montera MW, Mesquita ET, Colafranceschi AS, Oliveira Junior AC, Rabischoffsky A, Ianni BM, et. al. I Brazilian guidelines on myocarditis and pericarditis. Arq Bras Cardiol. 2013;100(4 Supl 1):1-36. DOI: https://doi.org/10.5935/abc.2013S004
21. Kutzner H, Argenyi ZB, Requena L, Rütten A, Hügel H. A new application of BCG antibody for rapid screening of various tissue microorganisms. J Am Acad Dermatol. 1998 Jan;38(1):56-60. DOI: https://doi.org/10.1016/s0190-9622(98)70539-0
22. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Coordenação-Geral de Vigilância das Doenças de Transmissão Respiratória de Condições Crônicas. Manual de recomendações para o diagnóstico e tratamento das Doenças causadas por micobactérias não tuberculosas no Brasil. Brasília (DF): Ministério da Saúde; 2021.
23. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/ IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J RespirCrit Care Med. 2007 Fev;175(4):367-416.
24. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020 Jul;56(1):2000535.
25. Dooley DP, Carpenter JL, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin Infect Dis. 1997 Out;25(4):872-87.
26. Isiguzo G, Bruyn ED, Howlett P, Ntsekhe M. Diagnosis and management of tuberculous pericarditis: what is new? Curr Cardiol Rep. 2020 Jan;22(1):2. DOI: https://doi.org/10.1007/s11886-020-1254-1
27. Marras TK, Daley CL. A systematic review of the clinical significance of pulmonary Mycobacterium kansasii isolates in HIV infection. J Acquir Immune Defic Syndr. 2004 Ago;36(4):883-9.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















