Cutaneous cryptococosis: systemic or localized disease?
DOI:
https://doi.org/10.5935/2764-734X.e20230324Keywords:
Cryptococcosis, Invasive Fungal Infections, Cutaneous Manifestations., Case ReportAbstract
Cutaneous cryptococcosis is a fungal infection that can manifest as localized or disseminated disease in both immunocompetent and immunocompromised patients. The present report describes a case of an 81-year-old man from the rural area of Bahia, with the cutaneous form of the disease. Initially the patient was considered to have primary cryptococcosis and was treated with fluconazole. However, in order to rule out disseminated disease, the patient underwent chest tomography, which showed pulmonary nodules that, associated with positive antigenemia, justified the change to liposomal amphotericin B. There was a satisfactory clinical response with no evident complications related to the treatment, but a late metastatic prostate cancer diagnosis allowed to question the supposed fungal infection spread. The differentiation between localized and disseminated cryptococcosis is difficult, but it is fundamental for the proper direction of treatment.
Downloads
INTRODUCTION
Cryptococcosis is one of the most common opportunistic fungal infections that affect immunosuppressed patients, being of subacute or chronic evolution, mainly compromising the central nervous system (CNS) and lungs1. Although these are the most common sites, other organs can also be affected, such as the skin2.
Cutaneous cryptococcosis (CC) occurs in both immunosuppressed and immunocompetent patients and can be classified as primary (due to direct inoculation of the fungus) or secondary (by hematogenous dissemination in patients with systemic disease)3,4. When CC is diagnosed as a primary disease, the identification of the fungus should be restricted to the skin, without evidence of systemic disease5, a fact that has direct implications for the treatment to be prescribed. The present report of a CC case exemplifies well the difficulty of distinguishing the localized from the systemic disease owing to the clinical findings and confounding complementary tests.
CASE REPORT
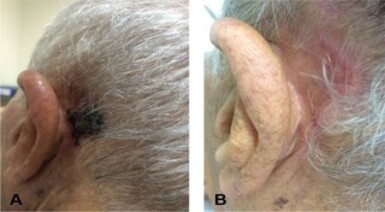
This study presented an 81-years-old man, born in Livramento de Nossa Senhora, a rural city in the state of Bahia, Brazil. The patient worked with corn and bean plantation and livestock. He sometimes visited his daughter in São Paulo, and on one of the trips he sought the Outpatient Clinic of Tropical Diseases and Zoonoses of the Emilio Ribas Institute of Infectology with a complaint of a left retroauricular nodular lesion persisting for four months, initially pruritic but progressed to pain, ulceration, purulent secretion, and formation of red crust two months ago (Figure 1A). A month earlier the patient had sought medical care in Bahia, when leishmaniasis was diagnosed by direct research on the secretion of the wound (sic) and treatment with glucantime 900 mg/day was started. From the fourth dose of this drug, the patient presented pain and tingling in the left arm, so he discontinued the medication on his own on the eighth day.
Figure 1. A. Left retroauricular lesion with ulcers on an erythematous base, covered by hematic crust; B. Healing of the lesion after treatment.
Skin biopsy of the retroauricular lesion was performed, and it showed diffuse histiocytic tissue reaction containing numerous yeasts; the fungus test was positive by Grocott’s staining and morphologically compatible with Cryptococcus spp. Direct screening and polymerase chain reaction for leishmaniasis in this biopsy were negative, as well as serology for histoplasmosis. Thus, specific treatment with fluconazole 400 mg every 12 hours was initiated. Other laboratory tests such as HIV serology, hormonal dosages, and diabetes testing were all negative or normal. Considering a possible systemic involvement due to fungal infection, a computer tomography scan of the skull (normal) was performed, followed by cerebrospinal fluid (CSF) collection for chemocytological examination, direct research, and cultures for fungi, all of them with negative results. Antigenemia for Cryptococcus spp. was reactive. Chest tomography, in turn, revealed multiple well-delimited pulmonary nodules in both lungs, in large numbers on the right, measuring up to 2.3 cm in their largest diameter (images not available).
Given this evidence of disseminated disease (positive antigenemia and presence of pulmonary nodules), the treatment was modified to liposomal amphotericin B on a day-hospital regimen, with intravenous infusion of the medication three times a week. After the cumulative dose of 3,685 mg, there was scarring of the lesion (Figure 1B) and a new antigenemia was negative - there are no records of complications related to the treatment.
After the end of treatment with amphotericin, fluconazole 600 mg/day was prescribed as an outpatient use for four months, when he began to complain of urine voiding effort and decreased caliber of the urine jet. Prostate ultrasonography was requested, which identified a significant increase in the prostate gland (58 × 55 × 49 mm3, with an estimated prostate weight of 83 g), accompanied by high dosage of prostate-specific antigen (PSA): 5.8 ng/mL (reference value <4 ng/mL). He was then referred to a specialized urology service, where he underwent transrectal biopsy that confirmed prostate adenocarcinoma (Gleason 6). The cancer treatment was based on the prescription of a hormonal inhibitor, and in the same service, the patient was admitted for bronchoscopy with negative direct stain and fungi culture in the bronchoalveolar lavage. The last radiological control (more than 5 years after the treatment of cryptococcosis) showed that the nodular lesions in both lungs were stable in terms of number and size (Figures 2A, 2B and 2C); in addition, a pathological fracture in the fourth thoracic vertebra (Figure 2D) was identified, which was later treated with palliative radiotherapy due to pain. Pulmonary nodules were thus considered secondary to neoplasia and not to fungal dissemination.
Figure 2. A, B and C. Chest tomography - arrows indicate multiple rounded solid nodules, in both lungs; D. Axial slice from a computed tomography showing fracture of the fourth thoracic vertebra (T4).
DISCUSSION
Cutaneous involvement in patients with disseminated cryptococcosis occurs in approximately 10-20% of cases and may present as the first manifestation of the disease as well as an early sign of dissemination6. The lesions are usually multiple and dispersed, located in areas hidden by clothing and on exposed skin.
On the other hand, the lesions of primary CC are usually solitary or confined to a limited area, most commonly in bare areas7,8, such as head or neck, manifesting different forms of presentation such as blisters, nodules, granulomas, acneiform papules, lesions similar to those of Molluscum contagiosum, pustules, ulcers, subcutaneous edema, abscess, cellulitis with eczematous plaque, and plaques3. Primary CC occurs in populations with different characteristics (age and gender) and immune status and is common in elder men, either immunocompetent or immunocompromised patients living in rural areas9.
Because of patient’s advanced age, the single lesion in the retroauricular region, and his origin from a rural area, the case was very suggestive from a clinical and epidemiological point of view as a primary form of the disease. But the necessary continuity of the investigation ended up questioning this diagnostic reasoning.
Histopathological examination and fungal culture of the tissue fragment sample are imperative for the diagnostic definition, as a range of differentials can mimic CC. Among the infectious causes, mycobacteriosis, Molluscum contagiosum, and other fungal infections stand out such as coccidioidomycosis, blastomycosis, and histoplasmosis10,11. Although clinically similar (our patient’s lesion was an ulcerated nodule), cutaneous leishmaniasis can also be considered as a differential diagnosis, but from a clinical and non-laboratory point of view. The diagnosis made in Bahia was the first confusion factor for the understanding and management of the case, even after prompt skin biopsy.
Although fungal infection may be confirmed, to determine the extent of infection, radiological investigation of the lungs and CNS, CSF collection and analysis (including fungal research and culture), and other methods, such as cryptococcal antigen measurement in the blood and other biological samples are mandatory12.
The radiological images of pulmonary involvement by cryptococcosis represent a challenge themselves owing to the various possible differential diagnoses. In immunocompetent patients, localized nodules and masses predominate (unilateral or bilateral, well defined, not calcified, rarely cavitated, typically subpleural in location, and ranging in diameter from 0.5 to 4.0 cm12). In immunocompromised patients, interstitial infiltrate and diffuse opacities are more frequent13. These radiological findings of cryptococcosis may mimic other clinical conditions such as community acquired bacterial pneumonia caused by Streptococcus pneumoniae or Pseudomonas spp., atypical pathogens (Mycoplasma pneumoniae or Chlamydia pneumoniae), mycobacteria, viral pneumonia (cytomegalovirus), other fungal diseases (aspergillosis, pneumocystosis, histoplasmosis, and blastomycosis), and primary or metastatic lung neoplasia14. Given the clinical and radiological suspicion, therefore, laboratory confirmation through microbiological studies and cultures and/or histological study (bronchoalveolar lavage and/or lung biopsy) should be carried out15. The fact is that the patient in this report presented bilateral pulmonary nodules that were not immediately and properly investigated, predominating the empirical and logical reasoning in favor of the disseminated fungal infection at first, mistaken in view of radiological evolution, maintained over the years of outpatient follow-up.
Another confounding factor in this case was positive antigenemia, that is, the detection of the capsular polysaccharide antigen of Cryptococcus spp. in the blood by the agglutination of latex, a test that can also be performed in urine samples, bronchoalveolar lavage, and CSF16. Antigen titration in blood of 1:4 is highly suggestive of cryptococcal infection and titers equal or higher than 8 usually indicate an active disease17. A positive result may reflect an increased risk of more severe localized or spread disease 18. However, in the series published by Neuville et al.19, seven of the 28 patients with localized disease had positive antigenemia in the blood, evidencing that even patients with localized disease can present positive antigenemia. Our patient presented positive antigenemia, but the method performed was qualitative and not quantitative; by this criterion alone, excluding or confirming the disseminated disease should not be reliable.
The recommended treatment of primary CC is based on the use of antifungal azole derivatives, particularly fluconazole at a dosage of 400 mg/day orally (or possibly intravenously in more severe cases), for both immunocompetent and immunosuppressed patients4. Although amphotericin B, 5-fluorocytosine, and other azoles, such as itraconazole and ketoconazole have also been reported for the treatment of primary CC17, its use (among others due to its nephrotoxicity) is more recommended for very extensive cases or for the treatment of disseminated cryptococcosis20. Mortality caused by disseminated cryptococcosis is estimated at about 10% in developed countries, reaching 43% in developing countries. Amphotericin B reduced these rates by approximately 30%12, which obviously justifies its use in selected cases.
CONCLUSION
This case report exemplifies the importance of considering infectious causes such as cryptococcosis among the differential diagnoses of chronic skin lesions, requiring proper diagnostic confirmation (preferably through histological study) to direct the specific treatment. Once the fungal infection is confirmed, in cases of CC, it is still mandatory to further investigate any systemic involvement in a comprehensive way, even in immunocompetent patients; in these cases, amphotericin becomes the recommended treatment, which can bring serious adverse effects, especially in the elderly patient population.
The diagnostic definition between primary CC and disseminated disease is not always easy. We conclude that the case reported here was of a localized cutaneous form of cryptococcosis, either because of its epidemiological history and clinical form or because of the negative search for fungi in the bronchoalveolar lavage and the late stability of the pulmonary nodules (associated with vertebral fracture) attributed to the diagnosis of prostate cancer kept under treatment.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Christanson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol J. 2003 Jun;41(3):177-88.
2. Du L, Yang Y, Gu J, Chen J, Liao W, Zhu Y. Systemic review of published reports on primary cutaneous cryptococcosis in immunocompetent patients. Mycopathologia. 2015 Aug;180(1-2):19-25.
3. Noguchi H, Matsumoto T, Kimura U, Hiruma M, Kusuhara M, Ihn H. Cutaneous cryptococcosis. Med Mycol J. 2019;60(4):101-7.
4. Marques SA, Bastazini Junior I, Martins AL, Barreto JA, Barbieri D’Elia MP, Lastória JC, et al. Primary cutaneous cryptococcosis in Brazil: report of 11 cases in immunocompetent and immunosuppressed patients. Int J Dermatol. 2012 Jul;51(7):780-4.
5. Ng WF, Loo KT. Cutaneous cryptococcosis – primary versus secondary disease: report of two cases and review of the literature. Am J Dermatopathol. 1993 Aug;15(4):372-7.
6. Tabassum S, Rahman A, Herekar F, Masood S. Cryptococcal meningitis with secondary cutaneous involvement in an immunocompetent host. J Infect Dev Ctries. 2013 Sep;7(9):680-5.
7. Murakawa GJ, Kerschmann R, Berger T. Cutaneous Cryptococcus infection and AIDS: report of 12 cases and review of the literature. Arch Dermatol. 1996 May;132(5):545-8.
8. Pema K, Diaz J, Guerra LG, Nabhan D, Verghese A. Disseminated cryptococcosis: comparison of clinical manifestations in the pre-AIDS and AIDS era. Arch Intern Med. 1994 May;154(9):1032-4.
9. Amaral DM, Rocha RC, Carneiro LE, Vasconcelos DM, Abreu MA. Disseminated cryptococcosis manifested as a single tumor in an immunocompetent patient, similar to the cutaneous primary forms. An Bras Dermatol. 2016 Sep/Oct;91(5 Suppl 1):S29-S31.
10. Mayers DL, Martone WJ, Mandell GL. Cutaneous cryptococcosis mimicking gram-positive cellulitis in a renal transplant patient. South Med J. 1981 Aug;74(8):1032-3.
11. Lima AM, Rodrigues MM, Reis CMS. Cutaneous cryptococcosis mimicking leishmaniasis. Am J Trop Med Hyg. 2018 Jan;98(1):3-4.
12. Moretti ML, Resende MR, Lazéra MS, Colombo AL, Shikanai-Yasuda MA. Consenso em criptococose-2008. Rev Soc Bras Med Trop. 2008;41(5):524-44.
13. Miller Junior WT, Edelman JM, Miller WT. Cryptococcal pulmonary infections in patients with AIDS: radiographic appearance. Radiology. 1990 Jun;175(3):725-8.
14. Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med Mycol. 2019;57(2):133-50.
15. Limper AH. The changing spectrum of fungal infections in pulmonary and critical care practice: clinical approach to diagnosis. Proc Am Thorac Soc. 2010;7:163-8.
16. Swinne D, De Vroey C. Detection of circulating capsular polysaccharide antigenfrom Cryptococcus neoformans. J Clin Microbiol. 1992 Sep;30(9):2521.
17. Hafner C, Linde HJ, Vogt T, Breindl G, Tintelnot K, Koellner K, et al. Primary cutaneous cryptococcosis and secondary antigenemia in a patient with long-term corticosteroid therapy. Infection. 2005 Apr;33(2):86-9.
18. Nunez M, Peacock Junior JE, Chin Junior R. Pulmonary cryptococcosis in the immunocompetent host: therapy with oral fluconazole: a report of four cases and a review of the literature. Chest. 2000 Aug;118(2):527-34.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003 Feb;36(3):337-47.
20. Yamaguchi H, Komase Y, Ikehara M, Yamamoto T, Shinagawa T. Disseminated cryptococcal infection with eosinophilia in a healthy person. J Infect Chemother. 2008 Aug;14(4):319-24.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















