Weil’s syndrome: from various organic dysfunctions to a favorable outcome
DOI:
https://doi.org/10.5935/2764-734X.e20240438Keywords:
Leptospirosis, Weil Disease, Acute Kidney Injury, Renal Dialysis, Case reportAbstract
Human leptospirosis is the most widely distributed zoonosis globally. In tropical countries, it is an endemic disease, and outbreaks coincide with rainy periods, especially in flooded areas. In its early stages, early recognition of leptospirosis is challenged by the nonspecific signs and symptoms, making it necessary to value related epidemiological data to consider this diagnostic hypothesis, and subsequently, prescribe effective antimicrobial treatment from the beginning. Evolution of leptospirosis to a second stage with pulmonary, hepatic, and renal dysfunction determines a more serious and less frequent form of the disease, known as Weil’s syndrome. Herein, we describe a classic case of Weil’s syndrome, whose early recognition and appropriate treatment led to a satisfactory clinical response and total recovery, despite the initial severity of the condition.
Downloads
INTRODUCTION
Human leptospirosis is the most widely distributed zoonosis globally1,2. In tropical countries, it is an endemic disease and outbreaks coincide with rainy periods, especially in flooded areas.(2) Although it has historically been characterized as a rural disease, it affects mainly urban populations, and some authors consider it a neglected tropical disease owing to its close relationship with poverty and lack of public interest in addressing it3.
Clinical forms of leptospirosis range from a nonspecific initial presentation, usually associated with mild hepatic and renal dysfunction, to severe hemorrhagic forms that lead to fulminant respiratory and renal failure, known as Weil’s syndrome, accounting for 5%-10% of cases2. Thus, we present the case of a patient treated in São Paulo (SP) who, in January, rapidly developed multiple organ dysfunctions that met the diagnostic criteria for Weil’s syndrome, but made an excellent clinical recovery as a result of early diagnosis and appropriate management of the disease.
CASE REPORT
A 32-year-old brown man who worked in the outskirts of the city and had an incomplete primary education, went to the emergency room complaining of fever, arthralgia, and generalized myalgia that has limited his mobility five days after the onset of symptoms. He developed a productive cough with hemoptysis, jaundice, and severe abdominal pain. He was tachydyspneic and exhibited signs of ventilatory distress; bilateral conjunctival suffusion and rubinic jaundice were noticeable. He reported having been exposed to stagnant water 10 days before the onset of the condition, while cleaning a water tank where he found a dead mouse. Once the clinical suspicion of severe leptospirosis or Weil’s syndrome was established, antibiotic therapy was initiated with ceftriaxone 2 g/day, and on that same day, he was referred to the intensive care unit (ICU) of a hospital that specializes in infectious diseases.
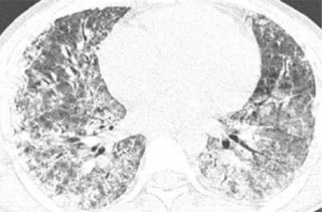
On admission to hospital, the patient was in serious medical condition, hypotensive, oliguric, and exhibited signs of respiratory distress. Thus, the laboratory tests described in Table 1 were requested. He soon progressed to ventilatory failure; subsequently, orotracheal intubation was performed. The imaging tests on admission were compatible with signs of alveolar hemorrhage and severe pulmonary involvement (Figure 1). However, there was no more exteriorization of blood through the airways. Moreover, still on the first day of hospitalization, emergency renal replacement therapy (RRT), namely hemodialysis, was initiated. Serology for leptospirosis (microagglutination test and enzyme-linked immunosorbent assay) was performed on the 10th day after the onset of symptoms; the result confirmed the diagnosis.
| Admission | D1 | D6 | D17 | D30 | |
|---|---|---|---|---|---|
| Serum creatinine (RV 0.66–1.25 mg/dL) | 5,9 | 5,6 | 4,4 | 3,1 | 1,1 |
| Serum urea (RV 19–43 mg/dL) | 109 | 132 | 140 | 115 | 34 |
| Serum potassium (RV 3.5–5.1 mmol/L) | 3,2 | 4,1 | 4,2 | 4,5 | 4,6 |
| Serum sodium (RV 137–145 mmol/L) | 130 | 140 | 139 | 137 | 143 |
| Hemoglobin (RV 13–18.0 g/dL) | 12,8 | 11,8 | 8,5 | ND | 9,0 |
| Leukocytes (RV 4000-11,000/mm³) | 15600 | 13200 | 25500 | ND | 7400 |
| Platelets (RV 140,000-450,000/mm³) | 52000 | 11000 | 85000 | ND | 319000 |
| Total Bilirubin / Direct Bilirubin (RV < 1.3/0.5 mg/dL) | 18,8 / - | 23,3 / 22 | 25,7 / 24,9 | 4,2 / 3,5 | 2,3 / 1,7 |
| Aspartate aminotransferase–AST (RV < 59 U/L) | 52 | 51 | 411 | 79 | ND |
| Alanine aminotransferase–ALT (RV < 50 U/L) | 39 | 36 | 103 | 47 | ND |
| Creatine phosphokinase (RV 35–170 U/L) | ND | ND | 9450 | ND | 43 |
Figure 1. Two chest computed tomography scans showing infiltrates consistent with diffuse alveolar hemorrhage.
He remained in intensive care and on daily hemodialysis for 14 days. With the reduction in ventilatory parameters, he was able to tolerate extubation on the 8th day of hospitalization, without any complications. Laboratory tests showed a drop in inflammatory and liver markers as well as a progressive improvement in renal dysfunction (Table 1). The patient was discharged on the 17th day of hospitalization still in the polyuric phase of renal recovery, having spent the last three days in a ward, without RRT He was referred for outpatient follow-up and was discharged from the nephrology department after a significant improvement in renal function markers proved in the last laboratory tests.
DISCUSSION
The case presented herein demonstrates a classic clinical presentation of leptospirosis with rapid progression (within a few days) to its severe form. It is of note that the symptoms in the early stages of the disease are nonspecific and can be confused with other diseases endemic in the country, such as dengue, chikungunya, yellow fever, spotted fever, and even self-limited flu-like illnesses. Suspicion arises mainly through the correlation of clinical data with the following epidemiological data: exposure to stagnant water, vulnerable living conditions, and living in the outskirts of a large city4,5. It is crucial to consider the diagnostic hypothesis of leptospirosis in these cases, as the initial phase of symptoms is when antimicrobial treatment is most effective, since it corresponds to the bacteremia phase1.
The development of Weil’s syndrome, however, is the critical phase when the diagnosis of leptospirosis is most commonly made, with the worst prognosis. Hepatic and pulmonary dysfunction, which is mainly due to inflammatory mechanisms attributed to the deposition of immune complexes and to direct invasion of the etiological agent into the vascular wall6, are determining factors for higher mortality7. However, the benefit of using antibiotics at this second stage is also well established, and they should anyway be started as soon as the disease is suspected2, as occurred in the present case.
In terms of pathophysiology, the peptidoglycan present in the cell wall of Leptospira spp. acts as an adhesion factor to the endothelium and leads to its dysfunction in conjunction with the release of TNF-alpha by peripheral mononuclear cells; high serum levels of this cytokine correlate with the severity of the disease8,9. The first vessels to be compromised are the capillaries, corroborating to the worsening of the liver, lungs, and kidneys. In the liver, disorganization of the hepatocyte arrangement is observed, caused by the loss of cadherins-glycoproteins expressed in the cell wall that determine cell-cell adhesion. Thus, direct cell damage is less important than loss of function, which explains the disproportionately high bilirubin relative to the value of transaminases7.
Ventilatory insufficiency with diffuse cotton-like infiltrates on chest computed tomography scan is explained by changes in the lung parenchyma also resulting from diffuse small vessel vasculitis that increases its permeability and allows extravasation of blood from the microvasculature into the interstitium. The clinical representation of these findings is described as diffuse alveolar hemorrhage (DAH). Macroscopically, there are focal hemorrhagic lesions and diffuse petechiae involving the parenchyma, pleura, and tracheobronchial tree, while microscopy shows the presence of interstitial edema9.
In 2010, Maroto et al.10 described the main predictive factors of pulmonary involvement in leptospirosis, with a perspective that an early assessment favors the rapid transfer of the patient to an ICU. In descending order of statistical relevance, these factors are as follows: hemodynamic instability in the first 24 hours; changes in mental state; increased serum potassium; increased serum creatinine; and increased respiratory rate. DAH has high mortality rates and requires aggressive management with mechanical ventilation support to supply oxygen under high levels of positive end-expiratory pressure in order to produce a pressure tamponade effect on pulmonary capillary bleeding11.
Moreover, acute kidney injury (AKI) in leptospirosis is characterized by the association of interstitial and tubular kidney damage. Both the direct nephrotoxic action of the leptospira and the action of immune response-inducing toxins are related to its genesis, while hemodynamic changes, jaundice, and rhabdomyolysis (characterized by muscle injury with an increase in creatine phosphokinase) also contribute to the mechanism of kidney damage12.The outer membrane of leptospira contains antigenic components responsible for tubular dysfunction and inflammation, which directly damage renal proximal tubular cells and increase the expression of proinflammatory genes/proteins12. In terms of renal pathology, the most common pattern is acute tubulointerstitial nephritis characterized by diffuse interstitial edema and mononuclear cell deposition. When the disease progresses to sepsis or distributive shock, another pattern of renal involvement is considered, which is acute tubular necrosis due to renal hypoperfusion13.
Classic clinical presentation of AKI caused by leptospirosis—polyuric or non-oliguric—is due to difficulty in urinary concentration secondary to increased resistance to the effects of vasopressin, leading to excess free water in the renal tubules. As in the present case, serum hypokalemia and hyponatremia in the initial phase of AKI is common. These changes can be explained by the decreased reabsorption of these electrolytes in the renal proximal tubule and the increased urinary flow, which is potentiated by exposure to high levels of aldosterone and cortisol, even in the presence of low glomerular filtration rate and increased serum creatinine13.
RRT for hemodynamic control should be started early and performed daily, as soon as the aforementioned risk factors are identified. This is the mainstay of treatment for severe forms of leptospirosis. This prioritization and early initiation of RRT has been described by Andrade et al.14 as having a significant effect on reducing mortality compared to the traditional hemodialysis regimen; the author emphasizes that the usual indications for the initiation of hemodialysis are not sufficient for the treatment of leptospirosis. The improved response obtained with daily RRT is similar to that described for other models of AKI with ATN in critically ill patients15,16. Kidney injury treated in this manner is usually reversible (the present case being an example) with no need for further RRT cycles after the acute condition has resolved.
CONCLUSION
This report describes a classic case of Weil’s syndrome that was correctly suspected on the basis of epidemiological data and that had a good outcome because of appropriate clinical management. In addition to antibiotic therapy, understanding the main pathophysiological mechanisms involved in the disease is crucial for making other assertive decisions, especially regarding intensive support for hemodynamic and ventilatory control and early initiation of daily RRT in severe forms of the disease.
"This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited."
References
1. Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731-40. DOI: 10.1016/S1474-4422(09)70134-6
2. Marra CM. Infectious and postinfectious vasculopathies. Neuroimaging Clin N Am. 2024;34(1):13-21. DOI: 10.1016/j.nic.2023.06.001
3. Maher MD, Douglas VP, Douglas KAA, Collens SI, Gilbert AL, Torun N, et al. Clinical and neuroradiologic characteristics in varicella zoster virus reactivation with central nervous system involvement. J Neurol Sci. 2022;437:120262. DOI: 10.1016/j.jns.2022.120262
4. Gilden D, Nagel M, Cohrs R, Mahalingam R, Baird N. Varicella zoster virus in the nervous system. F1000Res. 2015;4(Faculty Rev-1356):1356. DOI: 10.12688/f1000research.7153.1
5. Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system – Prognosis, diagnostics and treatment. J Infect. 2015;71(3):281-93. DOI: 10.1016/j.jinf.2015.06.004
6. Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27(3):356-60. DOI: 10.1097/WCO.0000000000000092
7. Bakradze E, Kirchoff KF, Antoniello D, Springer MV, Mabie PC, Esenwa CC, et al. Varicella zoster virus vasculitis and adult cerebrovascular disease. Neurohospitalist. 2019;9(4):203-208. DOI: 10.1177/1941874419845732
8. Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, Russman A et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77(4):364-70. DOI: 10.1212/WNL.0b013e3182267bfa
9. Gilden D. Varicella-zoster virus infections. Continuum (Minneap Minn). 2015;21(6):1692-703. DOI: 10.1212/CON.0000000000000246
10. Carod Artal FJ. Clinical management of infectious cerebral vasculitides. Expert Rev Neurother. 2016;16(2):205-21. DOI: 10.1586/14737175.2015.1134321
11. Kennedy PG, Mogensen TH. Determinants of neurological syndromes caused by varicella zoster virus (VZV). J Neurovirol. 2020;26(4):482-95. DOI: 10.1007/s13365-020-00857-w
12. Chiang F, Panyaping T, Tedesqui G, Sossa D, Costa Leite C, Castillo M. Varicella zoster CNS vascular complications. A report of four cases and literature review. Neuroradiol J. 2014;27(3):327-33. DOI: 10.15274/NRJ-2014-10037
13. Nagel MA, Niemeyer CS, Bubak AN. Central nervous system infections produced by varicella zoster virus. Curr Opin Infect Dis. 2020;33(3):273-8. DOI: 10.1097/QCO.0000000000000647
14. Cheng-Ching E, Jones S, Hui FK, Man S, Gilden D, Bhimraj A, et al. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci. 2015;351(1-2):168-73. DOI: 10.1016/j.jns.2015.02.017
15. Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58(11):1497-503. DOI: 10.1093/cid/ciu098
16. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16(2):12. DOI: 10.1007/s11910-015-0614-5
17. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde e Ambiente. Departamento de Imunizações e Doenças Imunopreveníveis. Manual dos Centros de Referência para Imunobiológicos Especiais [Internet]. 6ª ed. Brasília: Ministério da Saúde; 2023; [acesso em 31 de janeiro de 2024]. Disponível em: https://sbim.org.br/images/calendarios/manual-dos-centros-de-referencia-para-imunobiologicos-especiais-6a-edicao-2023.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















