Nosocomial infection by Cupriavidus metallidurans
DOI:
https://doi.org/10.5935/2764-734X.e20240945Keywords:
Ralstonia, Cupriavidus, Mass Spectrometry Matrix-Assisted Laser Desorption-Ionization, Health Care Associated Infection, Case Report.Abstract
A 68-year-old male patient remained in the intensive care unit for 75 days after surgery for drainage of intraparenchymal cranial hemorrhage and placement of an external ventricular drain. During his prolonged hospitalization, his prognosis was complex, with several complications and infectious episodes from various sources. During this period, Cupriavidus metallidurans (identified via the MALDI-TOF MS technique) was detected in two peripheral blood culture samples collected 26 days apart. In addition to the particularities of this bacterium, which is rarely isolated in the hospital environment, the review of this case led us to consider this agent as capable of causing active infection, which could have been a possible cause of the patient’s death.
Downloads
INTRODUCTION
Discovered in 1976, Cupriavidus metallidurans (CM) was previously called Ralstonia metallidurans. It is a gram-negative flagellated bacterium of the former genus Wautersia, with aerobic or facultative anaerobic metabolism, and notable for its ability to survive in environments with high concentrations of toxic heavy metals1-3. Difficult to identify in the laboratory, CM does not use sugars as a carbon source but prefers to use alcohol, carbon dioxide, and nitrogen gas to produce energy3. Because of these characteristics, it is considered extremophilic and is usually found in places such as mines, metallurgical plants, and chemical industries. Its name, in fact, derives from its resistance to copper4,5, leading some authors to call it a “metallophile”6. The nickname “modern alchemist” comes from its ability to precipitate tiny particles of solid 24-carat gold from gold tetrachloride present in the environment3.
There is evidence that infections in humans caused by Cupriavidus spp. are becoming more frequent, particularly in immunocompromised individuals and/or those with multiple comorbidities1,7-9. CM was first reported as a pathogen in 2011. The patient was a 74-year-old man with type 2 diabetes, arteriosclerotic heart disease, dyslipidemia, and hypertension who underwent subtotal pancreatectomy associated with splenectomy10. After multiple infections during the postoperative period, he developed sepsis and CM was detected in the blood culture. The nucleotide sequence of that strain of CM was identified and classified as H1130 and is deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) for future analysis and comparison5.
A search for the term “Cupriavidus” in the SCIELO database (https://www.scielo.br/) in August 2024, without time or language limits, retrieved 28 articles; however, none described an infection in humans. Similarly, a Boolean search using the terms “Cupriavidus AND infection” in the PUBMED database (https://pubmed.ncbi.nlm.nih.gov/) retrieved 110 publications, of which only four contained reports of CM infection in humans, none from Brazil.
Besides providing a brief review of the particularities of this bacterium, which is rarely isolated in the hospital environment, an analysis of this case led us to question its role as a mere contaminant or an agent capable of effectively causing healthcare-associated infections (HAIs), which may have been a possible cause of the patient’s death.
CASE REPORT
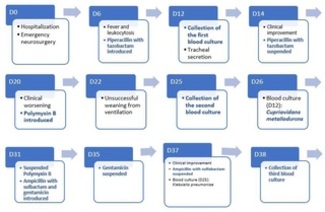
A 68-year-old White male patient was taken to the emergency department because of traumatic brain injury after falling from his own height, having tripped over a piece of furniture. On admission, the patient was agitated and had a decreased level of consciousness, requiring immediate orotracheal intubation. Computed tomography revealed an insular intraparenchymal cerebral hemorrhage on the left side with a hemoventricle. As a matter of urgency, surgery was performed for drainage and placement of an external ventricular drain on “day zero” (D0) of admission. He was immediately referred to the intensive care unit after surgery, where he received critical support through probes and catheters for mechanical ventilation, deep vein access, diuresis monitoring, and enteral feeding. He developed fever, leukocytosis, and elevated C-reactive protein levels, culminating in the extension of antibiotic therapy to include piperacillin and tazobactam on D6. On D12, the first pairs of blood cultures (from two sites, in aerobic and anaerobic vials) and urine and tracheal secretion samples were collected. Piperacillin and tazobactam were discontinued on D14 because of improvements in infection parameters. However, further clinical worsening led to the introduction of polymyxin B on D20, a decision based on the growth of Acinetobacter baumannii in the tracheal secretion. The positive results and respective antibiograms of the culture tests obtained during hospitalization are presented in Table 1.
| Type of sample | Date of collection | Date of result | Microorganism | Antibiogram | Note | ||
|---|---|---|---|---|---|---|---|
| Resistant | Intermediate | Sensitive | |||||
| Tracheal secretion | D12 | D19 | Acinetobacter baumannii | MDR(including meropenem and imipenem) | - | polymyxin BMIC: 64 µg/mL | polymyxin B was prescribed |
| Peripheral blood culture | D12 | D26 | Cupriavidis metallidurans (two samples) | piperacillin with tazobactamMIC <8 µg/mL | ceftazidimeMIC: 16 µg/mLciprofloxacinMIC > 1 µg/mLgentamicinMIC > 4 µg/mL | cefepimeMIC: 2 µg/mLimipenemMIC < 1 µg/mL | Considered contamination |
| Peripheral blood culture | D25 | D37 | Klebsiella pneumoniae | MDR | Trimetropim withsulfamethoxazole MIC: 4/76 µg/mL | - | No changes to medical prescription |
| Peripheral blood culture | D38 | D49 | Cupriavidis metallidurans (one sample) | ceftazidimeMIC > 32 µg/mLciprofloxacin MIC > 1 µg/mL gentamicinMIC > 4 µg/mL | - | cefepimeMIC < 1 µg/mLimipenemMIC < 1 µg/mLpiperacillin/tazobactamMIC < 8 µg/mL | cefepime and ertapenem were prescribed |
| Enterococcus faecalis(another sample) | levofloxacin, vancomycin, and teicoplanin | - | linezolidMIC = 2 µg/mLampicillinMIC = 4 µg/mL | No changes to medical prescription | |||
The result of the detection of CM in the first peripheral blood culture sample (identified by the MALDI-TOF MS method) was released only on D26 and was considered “contamination” by the local Hospital Infection Control Service (HICS). Echocardiography was performed on D28, which ruled out valve vegetation. The patient underwent tracheostomy on D30. On D31, the HICS changed the antibiotic to ampicillin with sulbactam associated with gentamicin, which was maintained for only 7 days considering the rapid hemodynamic stabilization and significant improvement in the tests. On D37, the result of another culture of peripheral blood collected on D25 revealed multidrug-resistant Klebsiella pneumoniae. Because there were no more clinical signs of active infection, the prescription was maintained without the need for new antibiotics. On D38, a pressure ulcer was identified in the left occipital region, which eventually progressed to the formation of an abscess in the left parietal region of the skull, next to the incisional scar from the operation performed on D0. This abscess was surgically drained on D50 after deep samples (including bone fragments) were collected for culture, but all samples were negative. Still on D50, a new antibiotic regimen was introduced with ertapenem and cefepime, a decision guided, this time, by the antibiogram of the CM identified again in a peripheral blood culture (collected on D38, 26 days after the first positive sample).
Notably, no CM was detected in any of the other 10 patients who were concurrently admitted to that unit (or at any other time throughout the hospital). The patient underwent gastrostomy on D56, the day before shift from the ICU to the ward. However, after just over 2 weeks, a new septic shock caused the death of the patient on D75. Figure 1 presents a schematic summary of the main events occurring throughout the hospital stay, from D0 to D75.
Figure 1. Main events related to the patient’s clinical and infectious evolution throughout hospitalization (D0 to D75).
DISCUSSION
Cupriavidus spp. are found in various environments, such as swimming pool water, soil, bottled mineral water from different brands, and even in the solutions used for nebulization1. A plausible explanation would be that taps are a potential source of contamination because they harbor biofilms that are difficult to remove4,11. This could enable bacterial transmission with a potential risk of infection, particularly in hosts with reduced immunocompetence. However, the identification of CM in the hospital environment is not common. In the present report, for example, if the source of infection was trivial, such as taps, more patients (all treated in the same environment and by the same team) could be expected to be contaminated or infected by CM; however, this was not the case. This argument allowed us to assume that the source of contamination must have been specific to this patient and not from an origin common to all inpatients in that unit10.
There have been reports of CM being identified in patients unrelated to the clinical complaint, for example, in the urine of a previously transplanted octogenarian with respiratory distress, a case understandably considered contamination2. Another evidence in favor of CM contamination is the case of a razor blade used to cut a paraffin block with biological materials. CM was unexpectedly identified at the molecular level in the histological sections12. However, it was later found that the other unused blades from the same batch-those that had not come into contact with the biological material being researched-also contained CM12. There is great genetic flexibility among these bacteria, which allows them to exchange plasmids and other elements. These genetic exchanges induce high mutation rates and changes in the O-antigen structure of their lipopolysaccharide membranes, which, in turn, helps them adapt to new environments and external media3,6. In other words, objects-particularly metals-can serve as fomites for CM. However, doubts remain regarding the role of these bacteria, i.e., whether they are mere contaminants or actually capable of causing active infection.
An Italian series of four patients considered infected by CM (and not just colonized) serves as a counter-argument, as it was reported that these patients could only be cured after removing the respective venous catheters and providing appropriate antibiotic therapy1. The presence of Cupriavidus spp. has also been cited as an opportunistic infectious agent in the bronchial secretion of patients with cystic fibrosis13,14. In the present report, the isolation of CM as a simple contamination came into question when it proved to be recurrent-even more so in a single patient from the ICU and in two peripheral blood samples collected at different times, 26 days apart. The hypothesis that hospital supplies and materials were contaminated was not supported. The evidence progressively reinforced the possibility that CM was one of the agents of HAI from an unknown focus of maintenance, facilitated by the presence of invasive catheters and probes for long periods.
Regarding CM resistance to antibiotics, the sensitivity profiles found in three other studies1,2,10 revealed resistance to gentamicin in all three and to ceftazidime in two. Piperacillin with tazobactam proved to be sensitive in all three publications, whereas imipenem was sensitive in only two. The data presented in Table 1 partially corroborates this information but does not prevent us from recommending avoiding the use of gentamicin and/or ceftazidime for the treatment of CM, prioritizing piperacillin with tazobactam or imipenem-which, like ertapenem, is from the carbapenem class. With the exception of the last regimen (introduced on D50), the choice of antibiotic therapy at various times throughout our patient’s prolonged hospitalization was not based on the cultures in which CM was detected but rather on the clinical and laboratory manifestations related to other foci and available antibiograms. Nevertheless, despite the broad spectrum of piperacillin and tazobactam in the fight against gram-negative bacteria, these antibiotics could not prevent reinfection (or recontamination) by CM because although the antibiogram of the first blood culture showed resistance, the third culture (collected on D38) proved to be sensitive. Also worth mentioning are ertapenem and cefepime-equally sensitive in the third sample-started on D50, which allowed for more lasting clinical improvement, although not definitive.
Finally, when traditional blood culture methods are used based only on the phenotypic characterization of the bacteria, more errors are expected in the laboratory identification of CM, even more so because it is rare10. Its growth in traditional culture media is relatively slow and can take up to >2 weeks, not including the time taken for phenotypic analysis15. The MALDI-TOF MS technique can enable the identification of this microorganism while it is still in the blood culture vial, as quickly as 15 min after enriching the material. Therefore, the use of mass spectrometry allows precise treatment and earlier execution, thereby reducing hospitalization time and cost and providing more favorable outcomes. In the present case, the use of MALDI-TOF MS also helped to give greater credibility to the identification of CM, particularly considering that both samples were collected at times of relative clinical improvement.
CONCLUSION
The identification of CM in the blood of a critically ill patient with several infectious foci, probes, and invasive catheters was initially considered contamination because this bacterium is rarely found in hospital environments. However, the growth of another strain of the same bacterium in another blood culture (collected from peripheral blood after 26 days) led the HICS team to review its clinical interpretation. Although the origin of the infection was not elucidated, CM was considered an agent that effectively caused HAI and probably contributed directly to the unfavorable outcome.
This was an isolated case in a single service, and no similar reports have been published in the Brazilian scientific literature. Having identified the microorganism using the MALDI-TOF MS saved time and improved the credibility of diagnosis. Regarding treatment, the antibiograms of the two samples showed sensitivity to cefepime and imipenem. The combination of piperacillin and tazobactam is another recommended option based on some international reports, but it did not prove effective in our case.
The authors thank all those who were directly or indirectly involved in carrying out this work, particularly the professionals at Miguel Couto Municipal Hospital and the Microbiology Center of the DASA laboratory.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. D'Inzeo T, Santangelo R, Fiori B, De Angelis G, Conte V, Giaquinto A, et al. Catheter-related bacteremia by Cupriavidus metallidurans. Diagn Microbiol Infect Dis. 2015 Jan;81(1):9-12. DOI: 10.1016/j.diagmicrobio.2014.09.015
2. Procop G, Church D, Hall G, Janda W, Koneman E, Schreckenberger P. The Nonfermentative Gram-negative Bacilli. In: Koneman's Color Atlas and Textbook of Diagnostic Microbiology. 7th ed. Philadelphia: Wolters Kluwer Health; 2017. p. 797-1059.
3. Notaro A, Vanacore A, Molinaro A, Speciale I. Structure and Conformation Study of the O-Antigen from the Lipopolysaccharide of Cupriavidus metal lidurans CH34. Polysaccharides. 2022; 3(1):188-99. DOI: 10.3390/polysaccharides3010009
4. Falkinham III JO. Introduction to Emerging Opportunistic Premise Plumbing Pathogens. In: Opportunistic Premise Plumbing Pathogens. Singapura: Jenny Stanford Publishing Pte Ltd; 2023. DOI: 10.1201/9781003321002
5. Monsieurs P, Provoost A, Mijnendonckx K, Leys N, Gaudreau C, Van Houdt R. Genome Sequence of Cupriavidus metallidurans Strain H1130, Isolated from an Invasive Human Infection. Genome Announc. 2013;1(6):e01051-13. DOI: 10.1128/genomeA.01051-13
6. Diels L, Van Roy S, Taghavi S, Van Houdt R. From industrial sites to environmental applications with Cupriavidus metallidurans. 2009;96(2):247-58. DOI: 10.1007/s10482-009-9361-4
7. Wauters G, Claeys G, Verschraegen G, De Baere T, Vandecruys E, Van Simaey L, et al. Case of catheter sepsis with Ralstonia gilardii in a child with acute lymphoblastic leukemia. J Clin Microbiol. 2001;39(12):4583-4. DOI: 10.1128/JCM.39.12.4583-4584.2001
8. Karafin M, Romagnoli M, Fink DL, Howard T, Rau R, Milstone AM, et al. Fatal infection caused by Cupriavidus gilardii in a child with aplastic anemia. J Clin Microbiol. 2010;48(3):1005-7. DOI: 10.1128/JCM.01482-09
9. Stovall SH, Wisdom C, McKamie W, Ware W, Dedman H, Fiser RT. Nosocomial transmission of Cupriavidus pauculus during extracorporeal membrane oxygenation. ASAIO J. 2010;56(5):486-7. DOI: 10.1097/MAT.0b013e3181f0c80d
10. Langevin S, Vincelette J, Bekal S, Gaudreau C. First case of invasive human infection caused by Cupriavidus metallidurans. J Clin Microbiol. 2011;49(2):744-5. DOI: 10.1128/JCM.01947-10
11. Santiago AJ, Burgos-Garay ML, Kartforosh L, Mazher M, Donlan RM. Bacteriophage treatment of carbapenemase-producing Klebsiella pneumoniae in a multispecies biofilm: a potential biocontrol strategy for healthcare facilities. AIMS Microbiol. 2020;6(1):43-63. DOI: 10.3934/microbiol.2020003
12. Sohrabi A, Norouzfar ZS, Eslamifar A, Arashkia A, Azadmanesh K. Isolation of Cupriavidus metallidurans from razor blade during paraffin embedded tissue sectioning. Clin Lab. 2011;57(7-8):641
13. Coenye T, Spilker T, Reik R, Vandamme P, Lipuma JJ. Use of PCR analyses to define the distribution of Ralstonia species recovered from patients with cystic fibrosis. J Clin Microbiol. 2005;43(7):3463-6. DOI: 10.1128/JCM.43.7.3463-3466.2005
14. Spilker T, Coenye T, Vandamme P, LiPuma JJ. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol. 2004;42(5):2074-9. DOI: 10.1128/JCM.42.5.2074-2079.2004
15. Pasternak J. New methods of microbiological identification using MALDI-TOF. Einstein (Sao Paulo). 2012;10(1):118-9. DOI: 10.1590/s1679-45082012000100026
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















