Paracoccidioidomycosis with cholestatic syndrome and duodenal involvement
DOI:
https://doi.org/10.5935/2764-734X.e20250352Keywords:
Paracoccidioidomycosis, Tuberculosis, Pulmonary, Cholestasis, Extrahepatic, Endoscopy, Gastrointestinal, Case ReportsAbstract
Paracoccidioidomycosis is an endemic systemic mycosis in Brazil, presenting as both acute and chronic forms, commonly associated with pulmonary infiltrates and exuberant skin and mucosal lesions. The intestinal form is rare, accounting for less than 1% of cases, and it can mimic diseases such as carcinoma, tuberculosis, and inflammatory bowel disease. We present an atypical case of paracoccidioidomycosis with duodenal involvement and cholestatic syndrome in a patient without known immunosuppression and with concomitant pulmonary tuberculosis, who showed a good response to specific treatment.
Downloads
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic mycosis caused by the thermodimorphic fungus Paracoccidioides spp., with P. brasiliensis and P. lutzii being the species responsible for the disease in humans. It is endemic to South America, Brazil having the highest number of cases reported in the literature1-4.
The chronic form of this disease is characterized by a prolonged latency period, with symptoms appearing decades after exposure to the fungus. The most commonly affected sites are the lungs, skin, and oral and nasal mucous membranes. However, the disease can spread to any tissue via lymphatic or hematogenous dissemination1-4.
In this report, we present the case of a patient without known immunosuppression who presented with gastrointestinal symptoms, obstructive cholestasis, and ulcerated lesions with granulomatous inflammation. Histological examination showed typical characteristics of PCM, thus confirming the etiological diagnosis. We emphasize the gastrointestinal involvement and the particularities of the endoscopic findings.
CASE REPORT
A 43-year-old man presented to the infectious diseases department with complaints of fever, night sweats, pain in the right hypochondrium, nausea, and weight loss of 10 kg in the previous 6 months. He reported that his condition worsened 7 days before admission because of diffuse abdominal pain, jaundice, choluria, and constipation. He had been undergoing treatment for pulmonary tuberculosis for 15 days (with rifampicin, isoniazid, pyrazinamide, and ethambutol), which had been diagnosed through a molecular test for Mycobacterium tuberculosis (without detection of rifampicin resistance genes) in bronchoalveolar lavage.
He was originally from northeast Brazil, where he had worked as a farmer, growing corn, beans, and rice. He lives now in Sao Paulo and has been working as a bricklayer for over 30 years. In addition, he mentioned a 3-year period of incarceration in the early 2000s. He consumed alcohol twice a week, was an ex-smoker (with a low tobacco load), smoked marijuana sporadically, and denied using crack, cocaine, or any intravenous drugs. There was no history of allergies or chronic illnesses.
On physical examination, his vital signs were normal. He was thin and jaundiced, with intact skin and no oral or nasal lesions. Cardiopulmonary auscultation showed no abnormalities. Abdominal palpation revealed pain in both costal margins, with hepatosplenomegaly of approximately 1 cm. No palpable peripheral lymph node enlargement was detected.
Laboratory tests showed the following: hemoglobin 12.1 g/dL, leukocyte count of 11,900/mm3, lymphopenia of 900/mm3, eosinophilia of 1,900/mm3, and platelet count of 638,000/mm3. The C-reactive protein level was elevated at 61.8 mg/dL. Transaminases were slightly increased (ALT 65 U/L, AST 55 U/L), whereas bile canaliculi enzymes (alkaline phosphatase 1,581 U/L, gamma-GT 1,526 U/L) and bilirubin (total 5.7 mg/dL, predominantly direct bilirubin 5.0 mg/dL) were significantly elevated. Serological tests for HIV, syphilis, and viral hepatitis (A, B, and C) were negative.
Abdominal computed tomography (CT) showed lymph node enlargement in the periaortic, mesenteric, peripancreatic, and retroperitoneal chains, forming conglomerates up to 5.0 cm in diameter, with liquefactive necrosis in the center (Figure 1). The bile duct was dilated (0.9 cm wide), and there was an abrupt reduction in its diameter near the hepatic hilum, suggesting extrinsic lymph node compression. Pulmonary CT showed a small area of consolidation surrounded by centrilobular micronodules at the left apex (Figure 2).
Figure 1. Contrast-enhanced computed tomography of the abdomen showing retroperitoneal, periaortic, and mesenteric lymph nodes in the upper abdomen forming conglomerates of up to 5 cm in diameter with a liquefied center (arrow).
Figure 2. Chest computed tomography shows centrilobular micronodules and small consolidations in the upper lobe of the left lung.
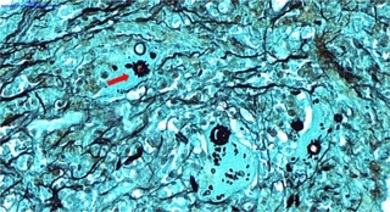
Upper digestive endoscopy revealed signs of chronic duodenitis in the second portion of the duodenum, associated with granulations with a mulberry-like appearance and ulcerated lesions on the mucosa with satellite friability, which were biopsied (Figure 3). Histopathological examination of the samples showed chronic granulomatous duodenitis with round-shaped fungal structures and multiple peripheral gemmules, typical of Paracoccidioides spp. (Figure 4). Ziehl-Neelsen staining did not show acid-fast bacilli, and the molecular test for Mycobacterium tuberculosis was negative (the endoscopic samples were not sent to the microbiology laboratory). There were no signs of malignancy.
Figure 3. Endoscopic images of the second portion of the duodenum showing well-defined, granulomatous, ulcerated lesions with an irregular erythematous component and central pallor. Biopsies were performed.
Figure 4. A. Histopathological examination of duodenal tissue with hematoxylin-eosin staining shows lymphohistiocytic infiltrate and granulomatous formations (arrow).
Figure 4. B. Grocott stain showing yeast cells with multiple exosporulation, typical of Paracoccidioides spp., inside a macrophage (arrow).
In addition, the serology of P. brasiliensis was performed using the double immunodiffusion agarose gel reaction, with the first titration of specific serum antibodies at 1:256. Based on the results, induction therapy was indicated, consisting of amphotericin B lipid complex associated with corticosteroids (1 mg/kg/day of prednisone) for 7 days. The tuberculostatic regimen was maintained. The patient tolerated the medication well and was discharged for outpatient follow-up after 8 days in the hospital with a prescription for 400 mg/day of itraconazole. He remained asymptomatic during follow-up appointments, with good adherence to treatment. After 18 months, the anti-PCM antibody titration had decreased to 1:8, there was a reduction and stabilization of abdominal lymph node size, the patient gained weight, and the duodenal lesions healed on control endoscopic examination. He is expected to continue treatment for at least another 6 months.
DISCUSSION
In this case, the epidemiological data were consistent with the literature regarding the predominance of PCM in men (with a male-to-female ratio of up to 13:1) and the history of exposure to rural life1,2. Co-infection with tuberculosis is also common, occurring before, simultaneously, or after the diagnosis of PCM in up to 20% of cases2.
Gastrointestinal involvement in PCM is rare; however, it can affect the entire digestive tract, from the mouth to the anus, and occurs most frequently in the pericecal region, where there is a higher concentration of lymphatic tissue3,4. The most frequent symptoms are abdominal pain and diarrhea1, which can mimic typical inflammatory bowel disease and even acute abdominal conditions, sometimes leading to bleeding episodes3.
Endoscopic findings related to PCM are rarely described in the literature and include intraluminal nodular lesions, ulcerations, stenosis, fistulas, and obstruction due to extrinsic lymph node compression4,5. Some reports have described the co-occurrence of these lesions with retroperitoneal abscesses and skin fistulas, thus mimicking other diseases such as Crohn’s disease6-8. Our patient had ulcerated lesions with raised, well-defined borders, mild signs of inflammation, and no signs of fistulization or perforation. This endoscopic appearance is nonspecific and allows tuberculosis, among other diseases, to be considered as a differential diagnosis1, highlighting the mandatory need for biopsy in such cases.
Jaundice can be caused directly or indirectly by pancreatic and intraluminal lesions associated with PCM; therefore, this fungal infection should also be considered in the differential diagnosis of cholestatic syndromes in compatible scenarios9. In this case, no lesions were visualized endoscopically near the duodenal papilla; therefore, biliary obstruction in the bile duct and the consequent cholestasis were attributed to extrinsic compression caused by peripancreatic lymphadenopathy, as described in other reports9. Endoscopic monitoring is also useful for anticipating complications such as fistulas and stenosis, as well as for evaluating treatment response4.
The reason for the initial induction treatment with amphotericin B in this patient was the severity criteria observed, notably weight loss of more than 10%, lymph node enlargement with cholestatic effects, and high serological titers1,2. For maintenance treatment (or treatment from the outset in less severe cases), itraconazole and the combination of sulfamethoxazole-trimethoprim (SMX-TMP) are the drugs of choice1,10. Studies have shown no difference in mycological control between these drugs, but itraconazole offers better adherence and a shorter treatment duration to reach cure criteria than SMX-TMP (9 months versus 18 months)10. However, SMX-TMP has several advantages, such as higher bioavailability and intravenous formulation10.
Treatment should be continued until body weight stabilizes, signs and symptoms are reduced, radiological findings improve, and, importantly, serum antibody titers fall1. A negative direct mycological test is typically obtained early in respiratory samples, but tissue lesions take longer to resolve. Integumentary lesions usually fade within 30 days, lymphadenopathies regress between 45 and 90 days, and radiological findings typically resolve or heal within 6 months1,2.
In this case, serial endoscopic follow-up was not performed. However, a single control performed after 18 months showed an adequate response to treatment, as no lesions remained in the previously affected areas. Additionally, there was a significant reduction in antibody titers, with serum anti-PCM antibody titers being the main parameter for monitoring treatment response1. Achieving a negative value (or stabilization at low values) can take up to 18 months1, with values of 1:2 (or 1:4 in specific cases) being considered a serological cure1.
Evidence supporting the use of corticosteroids in PCM is limited. The use of these agents should be considered in selected cases, such as central nervous system involvement, laryngeal stenosis prophylaxis, respiratory failure with an intense inflammatory response, and compression caused by an abdominal mass, as in the present case2. Surgical approaches may be necessary for patients with acute obstructive abdomen3.
CONCLUSION
This report highlights that PCM should be included in the differential diagnosis of extrinsic biliary obstructions caused by lymphadenopathy and ulcerated lesions with a granulomatous appearance in the digestive tract, even when tuberculosis is present, even in non-endemic areas. The findings also underscore the importance of always performing endoscopic biopsy in such cases. Long-term follow-up requires an individualized approach based on clinical, radiological, endoscopic, and especially serological monitoring, which is crucial for successful PCM treatment.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F, Kono ASG, Paniago AMM, et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop. 2017 Sep/Oct;50(5):715-40. DOI: 10.1590/0037-8682-0230-2017
2. Giron F, Vanegas M, Rodriguez LM, Hernandez-Santamaria V, Rey Chavez CE, Ortega J. Intestinal Paracoccidioidomycosis: Case report and literature review. Int J Surg Case Rep. 2022 Feb;91:106801. DOI: 10.1016/j.ijscr.2022.106801
3. da Cruz ER, Forno AD, Pacheco SA, Bigarella LG, Ballotin VR, Salgado K, et al. Intestinal Paracoccidioidomycosis: Case report and systematic review. Braz J Infect Dis. 2021 Jul/Aug;25(4):101605. DOI: 10.1016/j.bjid.2021.101605
4. Mantilla MJ, Africano F, Chaves JJ, Bolivar I, Tovar G. Paracoccidioidomycosis as a Cause of Ileocolonic Ulcers and Enterocutaneous Fistula. A Case Report. Acta Gastroenterol Latinoam. 2021;51(4):457-61. DOI: 10.52787/QCAJ6371
5. Healey S, Said W, Fayyaz F, Bell A. First report of paracoccidioidomycosis reactivation as a complication of immunosuppressive therapy for acute severe colitis in a caving enthusiast. BMJ Case Rep. 2020 Jul;13(7):e234125. DOI: 10.1136/bcr-2019-234125
6. Lomazi EA, de Negreiros LMV, Magalhães PVVS, Togni RCS, de Paiva NM, Ribeiro AF, et al. Intestinal paracoccidioidomycosis resembling Crohn's disease in a teenager: a case report. J Med Case Rep. 2018 Apr 30;12(1):108. DOI: 10.1186/s13256-018-1641-z
7. Brunaldi MO, Rezende REF, Zucoloto S, Garcia SB, Modena JLP, Machado AA. Co-infection with Paracoccidioidomycosis and human immunodeficiency virus: report of a case with esophageal involvement. Am J Trop Med Hyg. 2010 Jun;82(6):1099-101. DOI: 10.4269/ajtmh.2010.09-0751
8. Duani H, Nunes VR, Assumpção AB, Saraiva IS, Rosa RM, Neiva AM, et al. Bilateral paracoccidioidomycotic iliopsoas abscess associated with ileo-colonic lesion. Rev Soc Bras Med Trop. 2012 Oct;45(5):649-51. DOI: 10.1590/S0037-86822012000500021
9. Bernardes Filho F, Sgarbi I, Santos FSD, Sampaio RCR, Queiroz RM, Fonseca SNS, et al. Acute paracoccidioidomycosis with duodenal and cutaneous involvement and obstructive jaundice. Med Mycol Case Rep. 2018 Jan 12;20:21-5. DOI: 10.1016/j.mmcr.2018.01.005
10. Cavalcante RS, Sylvestre TF, Levorato AD, de Carvalho LR, Mendes RP. Comparison between itraconazole and cotrimoxazole in the treatment of paracoccidiodomycosis. PLoS Negl Trop Dis. 2014 Apr 17;8(4):e2793. DOI: 10.1371/journal.pntd.0002793
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















