Pituitary dysfunction associated with tuberculous meningitis
DOI:
https://doi.org/10.5935/2764-734X.e20250462Keywords:
Tuberculosis, Endocrine, Tuberculosis,, Central Nervous System, Hypophysitis, Case reportAbstract
Tuberculosis can lead to endocrine/metabolic changes in different glands, with varying clinical presentations and forms, while the incidence of pituitary involvement is unknown. We present the case of a patient with a recent diagnosis of AIDS who, upon presenting with tuberculous meningitis, developed diabetes insipidus, hypogonadotropic hypogonadism, and hyperprolactinemia. The diagnosis of hypophysitis was established, but there was no full recovery of hormonal function even after specific treatment.
Downloads
INTRODUCTION
Tuberculosis is a disease that can progress with endocrine and metabolic changes due to the involvement of any endocrine gland (adrenal, thyroid, and pituitary), with varying clinical manifestations that may mean exclusive local infection or reflect a para-infectious reaction from another affected site1. The incidence of the association between pituitary involvement and tuberculous meningitis is unknown, and there are no specific guidelines for its treatment1.
In the present case, the patient had a recent diagnosis of AIDS and tuberculous meningitis and progressed with hypophysitis, a presentation that added complexity to the clinical management; despite adequate treatment of tuberculosis, total recovery of glandular function was not achieved.
CASE REPORT
A 62-year-old man from Sao Paulo, with no history of comorbidities, reported a non-productive cough and weight loss that had been progressively worsening for 9 months. About 2 months ago, he began to experience fluctuations in his level of consciousness, mental confusion, episodes of syncope, difficulty walking, and holocranial headaches with no localizing signs. One month after his hospitalization, he was diagnosed with AIDS (positive serology for HIV with a CD4 lymphocyte count of 232 cells/mm3) and began treatment with antiretroviral drugs (ART), to which he adhered inconsistently. Physical examination on admission showed disorientation in time and altered gait (sensory ataxia).
The first exams carried out were computed tomography of the skull (whose findings showed slight and non-specific changes) and of the chest (which showed a residual calcium nodule in the middle lobe), with no signs of active tuberculosis. Subsequently, cerebrospinal fluid (CSF) was collected, which was clear, with the results being: cellularity 25/mm3 (97% lymphocytes and monocytes and 3% neutrophils), glucose 47 mg/dl, protein 103 mg/dl, lactate 27 mg/dl, negative acid-fast bacillus smear and rapid test for tuberculosis (Xpert MTB-RIF®), negative antigen test for Cryptococcus, while the late results of the cultures for bacteria and mycobacteria were also negative. The patient could not collect a sputum sample, but the lateral flow urine lipoarabinomannan assay returned positive. The latter result, combined with chronic meningoencephalitis, defined the diagnosis of probable tuberculous meningitis despite the negative pulmonary investigation; specific treatment was started with rifampicin, isoniazid, pyrazinamide, and ethambutol combined with dexamethasone 10 mg/day.
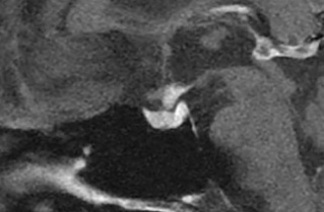
On the seventh day of hospitalization, however, the patient presented with polyuria, polydipsia, and hypernatremia, and the diagnostic hypothesis of diabetes insipidus was raised. To confirm the diagnosis, urine osmolality was measured on two occasions (197 and 180 mOsm/kg), and a therapeutic test with nasal desmopressin (10 mcg) was performed that increased urine osmolality from 134 to 205 mOsm/kg (52%). The results of the endocrinological investigation were as follows: serum follicle-stimulating hormone (FSH) 1.5 IU/L (reference value (RV) 0.9–11.9 IU/L); luteinizing hormone (LH) <0.2 IU/L (RV 0.6–12.1 IU/L); free testosterone 3 pmol/L (RV 131–640 pmol/L); sex hormone-binding globulin 132 nmol/L (RV 13.5–71.4 nmol/L); total testosterone <15 ng/dL (RV 247–674 ng/ml); thyroid-stimulating hormone (TSH) 3.57 mUI/ml (RV 0.4–4.5 mUI/ml) and free T4 (T4) 0.76 ng/dl (RV 0.7–1.8 ng/dl); insulin-like growth factor type 1 165 ng/ml (RV 75–212 ng/ml); and prolactin 49 ng/ml (RV 3.5–19.4 ng/ml). The established diagnosis was hypogonadotropic hypogonadism and hyperprolactinemia with no involvement of the thyrotropic and somatotropic axes. The corticotropic axis could not be assessed because the patient was on corticosteroids. Magnetic resonance imaging (MRI) of the pituitary gland (Figure 1) showed signs of hypophysitis of granulomatous etiology attributed to tuberculosis of the central nervous system. Once nasal desmopressin was restarted with therapeutic intent, the patient showed significant clinical improvement and, after a hospital stay of around a month, was discharged without neurological symptoms, with adequately controlled diuresis and normal serum sodium levels. His tuberculosis treatment ended after 12 months, but he still takes oral desmopressin and testosterone daily. He continues to be monitored on an outpatient basis in specialized infectious diseases and endocrinology care centers.
Figure 1. Sagittal T1-weighted magnetic resonance imaging slice (with contrast) showing thickening of the pituitary stalk, loss of signal in the posterior lobe (neurohypophysis), and involvement of the infundibulum.
DISCUSSION
The main causes of hypopituitarism are tumors, the effects of radiotherapy, and vasculopathy in general; more recently, infectious etiologies have been recognized2. Tuberculosis stands out as the most common infection associated with pituitary disease and can cause tuberculomas, vasculitis, and exudates in the sellar region, either by extension to the sphenoid sinus, brain or meninges or by hematogenous dissemination in the presence or absence of miliary disease2. Moreover, pituitary dysfunction can be an isolated manifestation or occur simultaneously in other sites affected by the disease1.
The clinical presentation of tuberculous hypophysitis is variable, ranging from asymptomatic or with subtle endocrine abnormalities to systemic symptoms or localized neurological complaints resulting from the enlargement of the gland (headaches and visual symptoms), which can occur in any part of the pituitary gland and hypothalamus1. The earliest hormonal dysfunctions are usually growth hormone deficiency and LH, FSH, and corticotropin (adrenocorticotropic hormone [ACTH]) deficiencies. In the case of a tuberculoma, deficiencies in ACTH, TSH, and hyperprolactinemia are most commonly reported2. In the present case, the patient had hypogonadism, hyperprolactinemia, and diabetes insipidus (less common).
Hypopituitarism associated with tuberculosis can occur years after the initial infection3. In a retrospective study in which pituitary function was evaluated in 49 adults with childhood tuberculous meningitis, 10 patients had functional abnormalities, none of whom had diabetes insipidus3. In another study conducted in India, 75 adults with recently diagnosed tuberculous meningitis had hyperprolactinemia (49.3%), cortisol insufficiency (42.7%), central hypothyroidism (49.3%), and multiple hormone deficiency (29%), also with no record of diabetes insipidus4.
Imaging exams are used to complement the diagnosis, especially MRI. The most common findings are atrophy or enlargement of the pituitary gland and hypothalamus and dilation of the third ventricle, as well as intrasellar tumors and thickening of the pituitary stalk with or without extension to the sphenoid sinus1, as was observed in our patient. In the aforementioned study, MRI revealed abnormalities in only 13.3% of cases, and there was no direct correlation between radiological abnormalities and hormonal dysfunction4. Sellar tuberculoma can be a confounding factor due to its similarity to adenomas, as reported in a study with a series of biopsies of patients with this other initial diagnostic hypothesis5.
Tuberculous meningitis is generally paucibacillary, with low counts in acid-fast bacillus smear and culture6. Xpert MTB-RIF has the highest (34,5%) diagnostic sensitivity in this context, versus 33.9% for Ziehl-Neelsen staining and 25.1% for culture7. This clinical reality reveals the absence ofa gold standard ofreference, and the present case is a good example of diagnostic limitation, since the rather non-specific hyperproteinorrhea, pleocytosis, and hypoglycorrhea were reinforced by the positive LAM result in the urine. Concomitant lung involvement occurs in only half of patients with neurotuberculosis8. It is important to realize that, unlike in meningeal forms, a diagnosis of tuberculous hypophysitis is only considered definitive if there is proper documentation of granulomas in the neural tissue; otherwise, it should only be considered presumptive1.
A study carried out in Zambia compared the diagnostic yield of LAM in urine and CSF to Xpert (only in CSF) in 550 adults with suspected tuberculous meningitis (86.2% of whom were people living with HIV). The respective sensitivity and specificity indices (considering CSF mycobacteria culture as the gold standard) were 24.1 and 76.1% for LAM in urine, 21.9 and 94.2% for LAM in CSF, and 52.9% and 94% for Xpert in CSF; these results demonstrated that the sensitivity of LAM in urine and CSF was low, but its specificity in CSF was similar to that of Xpert9.
There are scoring systems that can help clinical reasoning in the search for a diagnosis of tuberculous meningitis. One of these scores is the Lancet Consensus 10, in which 20 parameters are divided into 4 categories (clinical, CSF analysis, neuroimaging, and evidence of tuberculosis elsewhere)—the maximum possible score being 20. Although it was not effectively used in our case (since it was not mentioned in the medical records), the patient would have obtained 6 points because of the duration of symptoms >5 days and the presence of systemic signs, plus 4 points for the changes in the CSF, and another 4 points for evidence of tuberculosis elsewhere (positive LAM in urine), totaling 14 points that favor the diagnosis as “probable”10. Neuroimaging findings recommended for applying this score were not present in our case, namely hydrocephalus, meningeal enhancement, tuberculoma, infarction, and pre-contrast hyperdensity10.
The Brazilian Ministry of Health recommends the extension of the maintenance phase of the specific treatment for tuberculous meningitis to 10 months with isoniazid and rifampicin11. Corticosteroid therapy is recommended for the first 4–8 weeks, depending on the severity of the condition, with a gradual reduction in the dose over the following 4 weeks11. The start of ART should be delayed until the fourth or sixth week of tuberculosis treatment to avoid the adverse events of the immune reconstitution inflammatory syndrome12.
With regard to hormonal dysfunction, the response to treatment is variable, ranging from persistence of some specific insufficiency or even temporary panhypopituitarism13, 14, 15 to complete radiological and clinical resolution, even if only after 4 years16. Thus, inconsistent progression and therapeutic response are observed even in cases with a surgical approach or lesion resolution15.
CONCLUSION
The case described herein is an example of pituitary involvement associated with tuberculous meningitis, an uncommon phenomenon that adds further challenges in the context of AIDS. The manifestation of diabetes insipidus prompted a functional assessment of the pituitary—hypothalamic axis, and despite adequate clinical treatment for tuberculosis, full recovery of glandular function was not achieved, requiring multidisciplinary monitoring to control pituitary dysfunction.
References
1. Vinnard C, Blumberg EA. Endocrine and Metabolic Aspects of Tuberculosis. Microbiol Spectr. 2017 Jan;5(1):10.1128/microbiolspec.tnmi7-0035-2016. DOI: 10.1128/microbiolspec.tnmi7-0035-2016
2. Beatrice AM, Selvan C, Mukhopadhyay S. Pituitary dysfunction in infective brain diseases. Indian J Endocrinol Metab. 2013 Dec;17(Suppl 3):S608-11. DOI: 10.4103/2230-8210.123546
3. Lam KS, Sham MM, Tam SC, Ng MM, Ma HT. Hypopituitarism after tuberculous meningitis in childhood. Ann Intern Med. 1993 May 1;118(9):701-6. DOI: 10.7326/0003-4819-118-9-199305010-00007
4. Dhanwal DK, Vyas A, Sharma A, Saxena A. Hypothalamic pituitary abnormalities in tubercular meningitis at the time of diagnosis. Pituitary. 2010 Dec;13(4):304-10. DOI: 10.1007/s11102-010-0234-7
5. Ranjan A, Chandy MJ. Intrasellar tuberculoma. Br J Neurosurg. 1994;8(2):179-85. DOI: 10.3109/02688699409027964
6. Cherian A, Ajitha KC, Iype T, Divya KP. Neurotuberculosis: an update. Acta Neurol Belg. 2021 Feb;121(1):11-21. DOI: 10.1007/s13760-020-01575-0
7. Correia-Neves M, Fröberg G, Korshun L, Viegas S, Vaz P, Ramanlal N, et al. Biomarkers for tuberculosis: the case for lipoarabinomannan. Biomarkers for tuberculosis: the case for lipoarabinomannan. ERJ Open Res. 2019 Feb 11;5(1):00115-2018. DOI: 10.1183/23120541.00115-2018
8. Schaller MA, Wicke F, Foerch C, Weidauer S. Central Nervous System Tuberculosis: Etiology, Clinical Manifestations and Neuroradiological Features. Clin Neuroradiol. 2019 Mar;29(1):3-18. DOI: 10.1007/s00062-018-0726-9
9. Siddiqi OK, Birbeck GL, Ghebremichael M, Mubanga E, Love S, Buback C, et al. Prospective Cohort Study on Performance of Cerebrospinal Fluid (CSF) Xpert MTB/RIF, CSF Lipoarabinomannan (LAM) Lateral Flow Assay (LFA), and Urine LAM LFA for Diagnosis of Tuberculous Meningitis in Zambia. J Clin Microbiol. 2019 Jul 26;57(8):e00652-19. DOI: 10.1128/JCM.00652-19
10. Sulaiman T, Medi S, Erdem H, Senbayrak S, Ozturk-Engin D, Inan A, et al. The diagnostic utility of the “Thwaites’ system” and “lancet consensus scoring system” in tuberculous vs. non-tuberculous subacute and chronic meningitis: multicenter analysis of 395 adult patients. BMC Infect Dis. 2020 Oct 23;20(1):788. DOI: 10.1186/s12879-020-05502-9
11. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de recomendações para o controle da tuberculose no Brasil. 2a ed. Brasília: Ministério da Saúde; 2019 [acesso 2025 fev 20]. Disponível em: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/tuberculose/manual-de-recomendacoes-e-controle-da-tuberculose-no-brasil-2a-ed.pdf/view
12. Brasil. Ministério da Saúde. Secretaria de Ciência, Tecnologia, Inovação e Complexo da Saúde. Secretaria de Vigilância em Saúde e Ambiente. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos: Módulo 2: Coinfecções e Infecções Oportunistas [recurso eletrônico]. Brasília: Ministério da Saúde; 2023 [acesso 2025 fev 20]. Disponível em: https://www.gov.br/aids/pt-br/central-de-conteudo/pcdts/PCDT_HIV_Modulo_2_2024_eletrnicoISBN.pdf
13. Delsedime M, Aguggia M, Cantello R, Chiado Cutin I, Nicola G, Torta R, et al. Isolated hypophyseal tuberculoma: case report. Clin Neuropathol. 1988 Nov/Dec;7(6):311-3.
14. Srisukh S, Tanpaibule T, Kiertiburanakul S, Boongird A, Wattanatranon D, Panyaping T, et al. Pituitary tuberculoma: A consideration in the differential diagnosis in a patient manifesting with pituitary apoplexy-like syndrome. IDCases. 2016 Jul 29;5:63-6. DOI: 10.1016/j.idcr.2016.07.012
15. Tanimoto K, Imbe A, Shishikura K, Imbe H, Hiraiwa T, Miyata T, et al. Reversible hypopituitarism with pituitary tuberculoma. Intern Med. 2015;54(10):1247-51. DOI: 10.2169/internalmedicine.54.3435
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















