Intralesional vimblastine in oral Kaposi Sarcoma
DOI:
https://doi.org/10.5935/2764-734X.e202201008Keywords:
Sarcoma, Kaposi, Injectionsf, Intralesional, Herpesvirus 8, Human, HIV Infections, VinblastineAbstract
Kaposi’s sarcoma (KS) is a multicentric vascular neoplasm characterized by reddish or violet lesions on the skin, mucous membranes, and other sites. Associated with type 8 herpesvirus, it is more prevalent in men who have sex with men (MSM) and the mouth may be the only site involved. Oral KS treatments include surgical removal, radiation therapy, and intralesional chemotherapy. The objective of this case report was to document and evaluate the effectiveness of treating an oral KS lesion in a patient with AIDS using local injections of vinblastine solution (intralesional chemotherapy), aiming to highlight its positive results in a context of complementary treatment of the neoplasm.
Downloads
INTRODUCTION
Kaposi’s sarcoma (KS) is a multicentric vascular neoplasm that was first described in 1879 by Moritz Kaposi1. The first case of oral KS (classic variant) was described by Feit in 19282. The KS epidemic variant associated with HIV infection is strongly prevalent in men who have sex with men (MSM)3,4, being an AIDS-defining disease. KS may be the first clinical presentation of AIDS5, and because the mouth may be the only site involved6, patients with oral KS must necessarily be tested for HIV7. The palate is the most frequently affected region, and the gums are the second most frequent site5.
Oral KS treatments include surgical removal, radiation therapy, and intralesional chemotherapy. The total excision of the lesion often results in wounds that are difficult to heal, and radiotherapy can cause xerostomia and mucositis8. However, treatment with intralesional vinblastine is effective, with minimal toxicity; it is cheap and safe and can be repeated in case of recurrence6. This therapeutic modality has been recommended to treat the classical and African forms, with a positive response rate of 80%9. The objective of this case report was to document and evaluate the effectiveness of treating an oral KS lesion in a patient with AIDS using local injections of vinblastine solution (intralesional chemotherapy).
CASE REPORT
The patient was a white 25-year-old MSM born in São Paulo, SP, Brazil. In the previous 4 months, he had a weight loss of more than 6 kg, tiredness, evening fever (not measured), and progressive fatigue. He presented to the emergency room with worsening symptoms and was admitted to the intensive care unit owing to dyspnea in the context of the COVID-19 pandemic with tomographic involvement of the lungs greater than 50%. After ruling out SARS-CoV-2 infection, he was diagnosed with HIV/AIDS (CD4 cell count, 26 cells/mL; viral load, 695,000 copies/mL). Bronchoscopy findings were endoscopically normal and the bronchoalveolar lavage was inconclusive. A transbronchial biopsy was immunohistochemically positive for cytomegalovirus. Subsequently, lesions suggestive of oral KS were identified, and a biopsy of the right inguinal lymph node with immunohistochemistry confirmed the presence of herpesvirus type 8 or KS-associated herpesvirus (HHV-8–HVKS). A therapeutic antiretroviral regimen with tenofovir/lamivudine/dolutegravir was initiated as soon as the diagnosis of AIDS was established, starting systemic chemotherapy for KS 2 months after hospital admission.
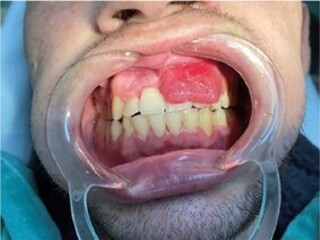
Two months after starting systemic chemotherapy against KS, the patient (already being followed up as an outpatient) complained of a single, nodular, raised, reddish oral lesion in the attached gum measuring 3 × 2 cm2, covering the dental crowns from the central incisor to the upper left canine (Figure 1), which was biopsied. Histological diagnosis was compatible with KS, and immunohistochemistry also confirmed the presence of HHV-8.
Figure 1. Initial lesion (D0).
The patient was invited to have his case documented prospectively for scientific and educational purposes, and after understanding and signing the informed consent form, he agreed to allow photographic documentation of his treatment according to the institutional protocol already well established (intralesional chemotherapy with vinblastine [Faulblastine®] 10 mg/10 mL at a dose of 0.1 mL/cm2 of lesion, administered every 7 days)5,6,8. Prospective follow-up lasted 70 days, with weekly photographic records and clinical observation of the volume and color of the lesion. There was also telephone contact with the patient 24 hours after each treatment to check for possible side effects. From the beginning of treatment, analgesics were provided for potential pain, if necessary. On the first day (D0) the patient received 0.1 mL of vinblastine solution. After 7 days (D7), a second dose of 0.4 mL was administered (Figure 2).
Figure 2. Site of lesion 7 days after the first treatment (D7).
After another 7 days (D14), a new dose of 0.4 mL was administered (Figure 3), and 7 days later (D21), a fourth dose of 0.3 mL was given (Figure 4). The total dose was 1.2 mL (1.2 mg).
Figure 3. Site of lesion 7 days after the second treatment (D14).
Figure 4. Site of lesion 7 days after the third treatment (D21).
The lesion showed total volume remission 14 days after the last treatment (D35) (Figure 5), but the patient was followed up every 7 days until 70 days after starting treatment (D70).
Figure 5. Site of lesion 14 days after the last treatment (D35), showing total remission of the lesion volume.
At the patient’s last follow-up appointment (D70), there was total remission of the lesion volume, although a violet color was still perceptible (Figure 6). During the entire treatment, no significant pain was reported, only minor discomfort the day after the first two treatments, with no need for analgesics.
Figure 6. Site of lesion 70 days after the first treatment (D70), showing total volume remission and almost total remission of lesion color.
DISCUSSION
KS has four epidemiological clinical variants: the classic, which is the first variant described in European and Mediterranean patients; the iatrogenic, which occurs in transplant patients; the endemic, African variant; and the epidemic variant, which is associated with HIV/AIDS1. KS is characterized by reddish or violet lesions on the skin, mucous membranes, and other sites10 and is associated with HHV-8–HVSK11, a class I carcinogenic virus as classified by the International Agency for Research on Cancer of the World Health Organization.
Oral KS may present as a single, macular, or elevated lesion, as well as with several lesions at different stages of development. In view of this, dentists should be able to recognize and know how to diagnose these oral lesions. The identification of viral DNA in the saliva of patients with oral KS suggests a possible transmission by oral secretions12.
The emergence of highly active antiretroviral therapy (HAART) had a significant positive impact on the immunological profile of HIV-infected patients, reducing the incidence and prevalence of several diseases, including KS. However, a late HIV diagnosis and/or non-adherence to treatment has made some of these diseases reappear, including KS again. In addition to HAART, several therapeutic alternatives have been associated with KS remission, like other antiviral drugs (such as foscarnet and ganciclovir) and immunotherapy. However, the prolonged use of some of these drugs can result in unwanted side effects (such as fever, neuropathy, nausea, and vomiting); therefore, their use is not always favorable in the long term. In parallel, the use of protease inhibitors has been proven effective against HIV-associated KS due to a direct relationship between clinical response and decreased HHV-8 viral load13.
Vinblastine is one of the chemotherapeutic agents of choice for the systemic (intravenous) treatment of KS. The highly alkaline nature of this drug is also believed to induce fibrosis when used locally8. Intralesional injections of vinblastine are commonly used to treat oral KS. The intralesional injection technique was widely described in the 1980s and 1990s, being accepted and used until now. The authors who described the technique to treat oral KS5,6,9 reported high success rates with no systemic side effects, good tolerance, and low cost, in addition to the possibility of repeating treatment in case of recurrence. Whenever reported, post-procedure pain is of short duration and low intensity and, therefore, easily controlled by analgesics.
CONCLUSION
The clinical case presented here describes a well-standardized therapeutic alternative for the treatment of a single exophytic oral KS lesion, with excellent results. In view of other therapeutic alternatives (surgical removal and radiotherapy, among others), positive aspects of intralesional treatment include the low dose of chemotherapy used (1.2 mg), fast response (total lesion volume remission in just 35 days after the beginning of the treatment, with four treatments), and minimal pain.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access artcle is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Karamanou M, Antoniou C, Stratigos AJ, Saridaki Z, Androutsos G. The eminent dermatologist Moriz Kaposi (1837-1902) and the first description of idiopathic multiple pigmented sarcoma of the skin. J BUON. 2013 Oct/Dec;18(4):1101-5.
2. Feit H. Sarcoma (Kaposi). Arch Derm Syphilol. 1928;18:611–2 apud Aroua A, Ali RB, Chokri A, Selmi J. Solitary Kaposi’s sarcoma in the oral cavity of an HIV-negative patient: a rare case report. Sch J Med Case Rep. 2017;5(11):713-6.
3. Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Invest. 2016 Sep;126(9):3165-75.
4. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990 Jan;335(8682):123-8.
5. Epstein JB. Treatment of oral Kaposi sarcoma with intralesional vinblastine. Cancer. 1993 Mar;71(5):1722-5.
6. Epstein JB, Lozada-Nur F, McLeod WA, Spinelli J. Oral Kaposi’s sarcoma in acquired immunodeficiency syndrome. Review of management and report of the efficacy of intralesional vinblastine. Cancer. 1989 Dec;64(12):2424-30.
7. Fatahzadeh M, Schwartz RA. Oral Kaposi’s sarcoma: a review and update. Int J Dermatol. 2013 Jun;52(6):666-72.
8. McCormick SU. Intralesional vinblastine injections for the treatment of oral Kaposi’s sarcoma: report of 10 patients with 2-year follow-up. J Oral Maxillofac Surg. 1996 May;54(5):583-9.
9. Volberding P, Conant MA, Stricker RB, Lewis BJ. Chemotherapy in advanced Kaposi’s sarcoma. Implications for current cases in homosexual men. Am J Med. 1983 Apr;74(4):652-6.
10. Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017 Sep;31(14):1903-16.
11. Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994 Dec;266(5192):1865-9.
12. Gorsky M, Epstein JB. A case series of acquired immunodeficiency syndrome patients with initial neoplastic diagnoses of intraoral Kaposi’s sarcoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Nov;90(5):612-7.
13. Lebbe C, Blum L, Pellet C, Blanchard G, Verola O, Morel P, et al. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi’s sarcoma. AIDS. 1998 May;12(7):F45-9.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















