Fusarium spp. as a cause of lung infection in a patient living with HIV/AIDS
DOI:
https://doi.org/10.5935/Infect_evidencia/e20220714Keywords:
Fusarium, HIV, Pneumonia, Glucocorticoids, Neutropenia, Critical Care, Case ReportAbstract
Fusarium is a genus of filamentous fungi that causes invasive disease in immunocompromised patients, especially those with hematologic cancer. AIDS immunosuppression has not been classically related to severe infection by this fungus. However, associated factors, such as prolonged neutropenia, use of corticosteroids, and T-cell deficiency, may predispose these patients to greater disease severity. This report describes a case of lung infection caused by Fusarium spp. in a patient living with HIV/AIDS after prolonged hospitalization in an intensive care unit due to multiple complications and co-infections.
Downloads
INTRODUCTION
Fusarium is a genus of filamentous fungi present in the environment, including organic matter and water. It can infect plants, animals, and humans. From a medical point of view, it has the potential to cause severe infection in immunocompromised patients, especially those with hematologic cancer1. To date, the literature presents few reports on Fusarium infection in people living with HIV/AIDS (PLWHA)2.
CASE REPORT
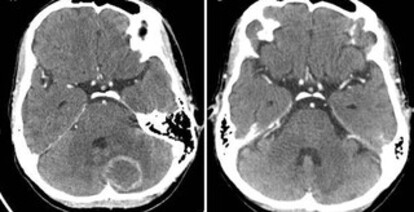
The patient was a 47-year-old woman living with HIV/AIDS for approximately 18 years. She presented a CD4 T lymphocyte count of 42/mm3 and HIV-1 viral load of 351,236 copies/mL (log 5.54) and had abandoned treatment more than a year prior-the last antiretroviral regimen used had been tenofovir, lamivudine, and atazanavir/ritonavir. She was admitted to a reference service with headache and vertigo. Contrast-enhanced cranial computed tomography (CT) revealed an expansive nodular lentiform and bilateral cerebellar formations with ascending transtentorial herniation, suggestive of cerebral toxoplasmosis (Figure 1A). Treatment was initiated on the first day of hospitalization with trimethoprim-sulfamethoxazole (320 mg of trimethoprim every 12 h for 44 days) and dexamethasone (4 mg every 6 h). The patient also had an ulcerated skin lesion at the frontal region that was recently biopsied at another service. A diagnosis of cutaneous leishmaniasis was made, and the specific treatment was initiated on the eighth day of hospitalization with liposomal amphotericin B (3 mg/kg) for 20 days. She was also prescribed fluconazole for the treatment of oral candidiasis during the first 11 days of hospitalization. On day 28 of hospitalization (coinciding with the suspension of amphotericin use), the patient presented general clinical worsening, with hypotension and fever, and sepsis with a pulmonary focus was suspected. She was medicated with piperacillin-tazobactam for 17 days. On the same occasion, the patient had diarrhea, an ulcerated lesion on the tongue, and decreased muscle strength in the lower limbs. A potential diagnosis of disseminated cytomegalovirus infection was considered; thus, a cerebrospinal fluid (CSF) sample was collected on day 28. A new cranial CT (before collection) showed improved toxoplasmosis lesions (a more evident finding on a new test performed on day 50, Figure 1B). CSF cytology showed no white blood cells, a red blood cell count of 3/mm3, a protein level of 17 mg/dL, and a glucose level of 36 mg/dL. Molecular rapid tests for tuberculosis and cultures for aerobic bacteria, fungi, and mycobacteria were all negative. Empirical treatment with ganciclovir (the molecular cytomegalovirus count test was unavailable) was initiated on day 29 and maintained for 21 days. On day 32, antiretroviral therapy with tenofovir, lamivudine, and darunavir/ ritonavir was restarted. The patient developed pancytopenia (hemoglobin level, 5.2 g/dL; red blood cell count, 1.78 million/mm3; neutrophil count, 750/mm3; and platelet count, 22 million/mm3), and her renal function worsened (creatinine level increased to 2.0 mg/dL). A peripheral blood sample collected on day 37 showed Salmonella sp. growth on culture (sensitive to and treated with ciprofloxacin, resistant to ampicillin and trimethoprim-sulfamethoxazole). The patient was transferred to the intensive care unit (ICU) on day 49. Chest CT showed parenchymal consolidations associated with ground-glass opacities in both lungs (Figure 2); thus, vancomycin was maintained from days 49 to 63 in addition to meropenem (already prescribed since day 45. In this context, she had marked renal function worsening with a need for dialysis (creatinine level of 4.91 mg/dL) while her leukopenia also worsened down to 200/mm3 on day 52.
Figure 1A. Skull CT with an oval image, annular enhancement by iodinated contrast in the cerebellar hemisphere, and associated edema. 1B: Skull CT showing improved lesions after 50 days of treatment for cerebral toxoplasmosis.
Figure 2. Chest CT showing the presence of parenchymal consolidations and ground-glass opacities.
The patient underwent bone marrow aspiration on day 57, which revealed hypocellularity in the three series, with intense macrophage activity; the cultures were negative. During that same week, the serum galactomannan level was 0.17 ng/ml (assessed by ELISA) and, in the search for other possible opportunistic agents, the patient’s peripheral blood showed Histoplasma capsulatum DNA amplification, but non-reagent double immunodiffusion serology both for histoplasmosis and paracoccidioidomycosis. New blood cultures were still positive for Salmonella sp., with a consistent sensitivity profile. Corticosteroid use was suspended on day 65. On day 69, the patient had a seizure and, for the first time, required orotracheal intubation. On day 72, a new chest CT showed marked lung improvement, with fewer diffuse opacities, interlobular interstitial thickening, and small pleural effusion on the left (Figure 3). Bronchoscopy was possible on day 80, showing small, reddish, friable, and bleeding plaques in the left bronchus and a granulomatous lesion covered by fibrinoid material in the right bronchus (images not available). Endobronchial biopsies were inconclusive: “presence of a leukocyte-fibrin plug, without evidence of fungal or mycobacterial structures.” A direct analysis (and later, culture) of mycobacteria in a bronchoalveolar lavage (BAL) was also negative; however, there was growth of a filamentous fungus, later identified in macro- and microcultures in Sabouraud agar and potato agar as Fusarium spp. Several blood, bone marrow, and tracheal secretion samples had not shown fungal growth until then.
Figure 3. Chest CT showing fewer ground-glass opacities, interlobular interstitial thickening, and left pleural effusion.
Due to the identification of Fusarium spp. in the BAL of a patient with criteria for probable fungal pneumonia in addition to the severity of the case as a whole, liposomal amphotericin B (3 mg/kg/day) was reintroduced on day 88 and maintained for another 30 days. However, there was no clinical improvement, particularly of the neurological aspects, which was further aggravated by recurrent airway bleeding after day 104 and by a new health care-associated infection (HAI). The patient dyed 121 days after hospital admission.
DISCUSSION
There are more than 300 species of Fusarium spp., although few are considered infectious for humans - F. solani and F. oxysporum are the most frequent complexes. The seasonality of these infections is related to rainy periods, in which fungus dispersion is increased by wind and humidity.3, 4
In general, immunocompetent individuals have a localized form of the disease, most commonly keratitis and onychomycosis. However, there are reports of arthritis, osteomyelitis, endophthalmitis, sinusitis, pneumonia, and even fungemia, among others4. In these patients, the main forms of infection are through trauma (direct inoculation) or through the use of contaminated contact lenses, although it may be related to other indirect factors, such as prolonged corticosteroid therapy5.
Low host immunity is a major factor in pathogen invasiveness as cellular response plays an important role in preventing the spread of the disease. Neutrophils play a relevant role in antifungal control; therefore, neutropenia is one of the main predisposing factors3. In immunosuppressed patients, infection occurs mainly through the respiratory route, although it can also happen through other forms already mentioned: a history of onychomycosis can be a fungus dissemination factor5. As it has already been isolated from hospital water sources, Fusarium can even affect immunocompromised hospitalized patients through the inhalation of aerosolized spores during bathing3.
Invasive disease has been reported, especially in patients diagnosed with acute myeloid leukemia or those undergoing allogeneic hematopoietic stem cell transplantation1, 4. The incidence of the disease in other immunosuppressed groups has not yet been well-established, with only occasional reports in solid organ transplant recipients and, even less frequently, in patients with HIV/AIDS4. In the case reported here, the patient had severe neutropenia for at least eight days, when she had been using corticosteroids for more than 40 days and was no longer receiving antifungal drugs. The association of these factors possibly made the Fusarium spp. infection more impactful than the HIV infection itself.
Clinical presentations of fusariosis in immunosuppressed patients are diverse, ranging from cutaneous or sinus involvement to disseminated disease forms3. Although usually associated with the disseminated form of the disease, isolated lung involvement can also occur1, in addition to allergic bronchopulmonary forms and hyper-sensitivity pneumonitis4. Fusarium has respiratory transmission, although pneumonia can also occur by hematogenous dissemination through a skin entry point, such as onychomycosis4. Disseminated skin lesions can also have a “metastatic” infectious origin, not necessarily being the source of infection4. The most frequent radiological findings in the lungs are interstitial or alveolar infiltrate, nodules, and cavitations1, 4, 6, 7. In this report, the patient had bilateral consolidations and ground-glass opacities.
Unlike other filamentous fungi, especially Aspergillus, the growth of Fusarium spp. in blood culture is often positive probably due to the circulation of yeast-like structures by occasional sporulation in the blood1. Morphologically, Fusarium hyphae are similar to those of Aspergillus: hyaline, with an acute septation angle but with the presence of “banana-shaped” macro- and microconidia of 2 3 μm8. The genera is differentiated by characteristics in macro- and microcultures, although the only safe identification method for Fusarium species is molecular testing9. In our case, this molecular test was not performed, although such taxonomic identification does not affect the therapeutic approach, since there are no data to date corroborating diferences between species regarding the response to antifungal agents.
According to the latest European consensus for the diagnosis of invasive fungal diseases10, the criteria for the definitive diagnosis of fusariosis include compatible anatomopathological findings (biopsy), sterile site culture (filamentous fungi; airway and urine samples are excluded), blood cultures, and nucleic acid amplification in tissue samples10. Considering that the lung sample obtained using transbronchial biopsy showed no fungal structures and that the blood culture was negative, evident risk factors for invasive disease (neutropenia, use of corticosteroids, and T-cell deficiency) associated with the growth of Fusarium spp. in BAL only classify the case reported here as a probable fungal lung disease10.
The criteria for probable fungal lung disease are different from those for a definitive diagnosis, considering the host’s clinical condition and characteristics and evidence of the fungus in collected samples10. Host-related factors include a prolonged use of corticosteroids (criterion of a dose greater than or equal to 0.3 mg/kg for at least 3 weeks in the previous 60 days) and T-cell deficiency. However, neither is the last factor not specific to PLWHA, nor has a median CD4+ lymphocyte count been defined10. The definitions by Donnelly et al.10 also do not cover critically ill patients admitted to the ICU, with scarce consistent data in the literature on the diagnosis of fusariosis in critically ill patients other than those affected by hematologic diseases11 .
There are few reports in the literature on Fusarium spp. infection in PLWHA, eight of which have been summarized in Table 112, 13, 14, 15, 16, 17, 18, 19. Four of those patients also had malignant neoplasms12, 14, 15, 16, and of the other four, only two were neutropenic13, 15. Regarding the infection site, there were two cases of disseminated disease13, 15, two with mucosal or cutaneous involvement13, 17, two with bloodstream infections12, 17, one with isolated lung infection16, and one with lymph node infection19. Two patients died, with both having disseminated disease and neutropenia; one of them had cancer13, 15. In the reported case of isolated lung infection16, fungus isolation was only possible through BAL, similar to what we reported here, without fungus identification in a biopsy fragment. Martino et al. reported two other cases not included in Table 1 due to the unavailability of most clinical data, with only the infection site (one case of endocarditis and one of lung infection) having been provided2.
| Reference (year) | Age (years) | CD4+T lymphocyte count (cell/mm3) | Neutrophils (cell/mm3) | Concomitant malignant disease | Corticosteroid use | Disease site | Species | Diagnostic sample | Treatment | Treatment time | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 (1996) | 44 | 6 | 1,040 | Kaposi's sarcoma | Not informed | Port-a-cath catheter infection | Fusarium oxysporum | Blood culture | Liposomal amphotericin B and granulocyte colony stimulator | 14 days (until death) | Death from Mycobacterium kansasii infection |
| 12 (1996) | 51 | Not informed | 200 | Absent | Not informed | Disseminated disease with endophthalmitis | Fusarium spp. | Vitreous humor, blood, liquor, and urine culture | Amphotericin B and fluconazole | 14 days (until death) | Death from disseminated fungal disease (brain, lungs, kidneys, thyroid, and lymph nodes) |
| 13 (1999) | 29 | 8 | 1,640 | Non-Hodgkin's lymphoma | Yes | Oral ulcer | F. solani | Biopsy and culture | Liposomal amphotericin B | 10 days (until death) | Death by NHL |
| 14 (2000) | 50 | Not informed | 150 | Non-Hodgkin's lymphoma | Not informed | Disseminated disease | F. solani and F. verticillioides | Skin biopsy and peripheral blood culture | Amphotericin B | 9 days (until death) | Death from disseminated fungal disease |
| 15 (2006) | 56 | 6 | No neutropenia | Bladder cancer (treatment refused) | Yes | Pulmonary | Fusarium spp. | Bronchoalveolar lavage microscopic examination and culture | Voriconazole | 6 days (until death) | Death from M. avium complex infection |
| 16 (2013) | 44 | 64 | 10,400 | Not informed | Yes | Bloodstream infection and endocarditis | F. solani | Blood culture | Not informed | Not informed | Deceased |
| 17 (2018) | 40 | 38 | No neutropenia | Absent | No | Disseminated cutaneous infection | F. solani | Skin lesion biopsy and culture | Itraconazole | 4.5 months | Recovered |
| 18 (2018) | 27 | 106 | 2,200 | Absent | No | Submandibular lymph node | F. keratoplasticum | Molecular investigation of lymph node aspirate | Liposomal amphotericin B and voriconazole | 30 days | Recovered |
The treatment of invasive or disseminated forms of fusariosis is based on systemic therapy with amphotericin B (lipid presentation) or voriconazole6, 20. Posaconazole and terbinafine also show antifungal activity20. Some cases require combined therapy6, 21. Amphotericin B deoxycholate has greater toxicity, in addition to having a worse outcome in multivariate analysis20. Echinocandins show no activity against Fusarium spp.1. If possible, induced immunosuppression should be reduced4.
In addition to being risk factors, neutropenia (if persistent at the end of treatment) and the use of corticosteroids are predictors of a worse prognosis20, 22. Mortality is high in the disseminated and lung forms, reaching up to 80% in patients1, 6, which can be observed in the PLWHA cases described in Table 1. Including this report, there are seven deaths out of nine cases. However, other complications and infections (such as cerebral toxoplasmosis, salmonellosis, repeated episodes of HAI, and our patient’s dialysis renal failure) commonly presented by these patients should be considered as either markers of the worst immune response or as aggravating factors of an already quite unfavorable condition.
CONCLUSION
HIV immunosuppression is not considered a risk factor for invasive filamentous fungal disease. However, its association with other more classic factors, such as neutropenia and the use of corticosteroids, deserve attention and diagnostic guidance aimed at specific and timely treatment.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Atribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007; 20(4):695-704.
2. Martino P, Gastaldi R, Raccah R, Girmenia C. Clinical patterns of Fusarium infections in immunocompromised patients. J Infect. 1994; 28 Suppl 1:7-15.
3. Galimberti R, Torre AC, Baztán MC, Rodriguez-Chiappetta F. Emerging systemic fungal infections. Clin Dermatol. 2012; 30(6):633-50.
4. Nucci F, Nouér SA, Capone D, Anaissie E, Nucci M. Fusariosis. Semin Respir Crit Care Med. 2015; 36(5):706-14.
5. Batista BG, Chaves MA, Reginatto P, Saraiva OJ, Fuentefria AM. Human fusariosis: An emerging infection that is difficult to treat. Rev Soc Bras Med Trop. 2020; 53:e20200013. https://doi.org/10.1590/0037-8682-0013-2020
6. Muhammed M, Anagnostou T, Desalermos A, Kourkoumpetis TK, Carneiro HA, Glavis-Bloom J, et al. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine (Baltimore). 2013; 92(6):305-16.
7. Marom EM, Holmes AM, Bruzzi JF, Truong MT, O’Sullivan PJ, Kontoyiannis DP. Imaging of pulmonary fusariosis in patients with hematologic malignancies. AJR Am J Roentgenol. 2008; 190(6):1605-9.
8. Guarro J, Gené J. Fusarium infections. Criteria for the identification of the responsible species. Mycoses. 1992; 35(5-6):109-14.
9. Summerell, BA. Resolving Fusarium: current status of the genus. Annu Rev Phytopathol. 2019; 57:15.1–15.17
10. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020; 71(6):1367-76.
11. Giacobbe DR, Cortegiani A, Karaiskos I, Mercier T, Tejada S, Peghin M, et al: The Fundicu Investigators. Performance of Existing Definitions and Tests for the Diagnosis of Invasive Fungal Diseases other than Invasive Candidiasis and Invasive Aspergillosis in Critically Ill, Adult Patients: A Systematic Review with Qualitative Evidence Synthesis. J Fungi (Basel). 2021; 7(3):176. https://doi.org/10.3390/jof7030176.
12. Eljaschewitsch J, Sandfort J, Tintelnot K, Horbach I, Ruf B. Port-a-cath-related Fusarium oxysporum infection in an HIV-infected patient: treatment with liposomal amphotericin B. Mycoses. 1996; 39(3-4):115-9.
13. Glasgow BJ, Engstrom RE Jr, Holland GN, Kreiger AE, Wool MG. Bilateral endogenous Fusarium endophthalmitis associated with acquired immunodeficiency syndrome. Arch Ophthalmol. 1996; 114(7):873-7.
14. Paugam A, Baixench MT, Frank N, Bossi P, de Pinieux G, Tourte-Schaefer C, et al. Localized oral Fusarium infection in an AIDS patient with malignant lymphoma. J Infect. 1999; 39(2):153-4.
15. Guarro J, Nucci M, Akiti T, Gené J. Mixed infection caused by two species of Fusarium in a human immunodeficiency virus-positive patient. J Clin Microbiol. 2000; 38(9):3460-2.
16. Tascini C, Ferranti S, Leonildi A, Menichetti F. Breakthrough Fusarium sp probable pneumonia during fluconazole therapy in an AIDS patient with diabetes, candidemia, Pneumocystis carinii pneumonia and cytomegalovirus disseminated infection. J Chemother. 2006; 18(2):227-8.
17. Esnakula AK, Summers I, Naab TJ. Fatal disseminated fusarium infection in a human immunodeficiency virus positive patient. Case Rep Infect Dis. 2013;2013:379320. https://doi.org/10.1155/2013/379320
18. Kumari I, Singh SK, Chauhan RK, Kaushal SK. Disseminated cutaneous fusariosis in human immunodeficiency virus-infected patient and dramatic response with oral itraconazole. Indian J Dermatol Venereol Leprol. 2018; 84(3):362-8.
19. Medaglia AA, Marco-Hernández J, de Ossó Acuña JT, Hermida Lama E, Martínez-Rebollar M, Caballero M, et al. Fusarium keratoplasticum infection in an HIV-infected patient. Int J STD AIDS. 2018; 29(10):1039-42.
20. Nucci M, Marr KA, Vehreschild MJ, de Souza CA, Velasco E, Cappellano P, et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect. 2014; 20(6):580-5.
21. Nucci M, Anaissie E. How we treat invasive fungal diseases in patients with acute leukemia: the importance of an individualized approach. Blood. 2014; 124(26):3858-69.
22. Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, et al. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003; 98(2):315-9.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















