Histoplasmosis: differentiation from tuberculosis in the context of AIDS
DOI:
https://doi.org/10.5935/2764-734X.e20230119Keywords:
Histoplasmosis, AIDS-related opportunistic infections, Acquired immunodeficiency syndrome, Diagnosis, Differential, Case reportAbstract
Histoplasmosis and tuberculosis are highly endemic in Brazil and have similar clinical and radiological manifestations, which can lead to diagnostic errors. In this report, we describe the case of a patient living with human immunodeficiency virus (HIV) for 12 years, who abandoned treatment for two years, and was presumptively diagnosed and treated for tuberculosis six months ago. However, due to the worsening of his symptoms, he was hospitalized and diagnosed with febrile wasting syndrome associated with severe sore throat, skin lesions, nodules in the larynx, and miliary micronodular pulmonary infiltrate. The diagnosis of histoplasmosis was confirmed by identifying the fungus in the biopsies, culturing clinical specimens, and detecting serum antibodies. The patient responded well to treatment with amphotericin B and was discharged on itraconazole and antiretroviral therapy.
Downloads
INTRODUCTION
Histoplasmosis may present as a disseminated disease in immunosuppressed patients1. Considering its high, albeit underestimated endemicity in Latin America and high lethality when associated with acquired immunodeficiency syndrome (AIDS)2,3, healthcare teams must effectively diagnose histoplasmosis and consider this infection among the main differential diagnoses of opportunistic diseases. However, patients with histoplasmosis manifest nonspecific symptoms, such as fever, weight loss, cough, and dyspnea, thereby, complicating the diagnosis of this disease, which is often confused with tuberculosis (TB)2,4-6. In the case reported below, this difficulty was the reason why the diagnosis of histoplasmosis was delayed for six months after the onset of symptoms, even in tertiary referral hospitals, meanwhile TB treatment was presumptively started, due to the common clinical and radiological characteristics of the two diseases.
CASE REPORT
The patient was a 37-year-old man who had been diagnosed with HIV infection 12 years ago and who had discontinued antiretroviral therapy (ART) in the last two years, without previous opportunistic diseases. He was a biologist with an epidemiological history of trails in caves and grottoes inhabited by bats, most recently approximately two years ago.
The patient presented with a weight loss of 14 kg in the last six months, associated with a fever of up to 39 ºC, night sweats. He developed dry cough and progressively worsening sore throat four months ago, at which time he sought care at a tertiary-level hospital. He was hospitalized for examination and diagnosed with TB based only on the clinical and radiological evidence, albeit without microbiological identification, subsequently initiating TB treatment. However, the symptoms worsened in the following two months, with an increase in cough and worsened sore throat, in addition to dysphagia (he was no longer able to swallow solids), hoarseness, dyspnea, and onset of frontal headache. Due to such worsening, he sought care at a referral hospital for infectious diseases in the city of São Paulo.
Upon arrival at the emergency room, the patient was observed to be normotensive, tachycardic, and dehydrated. Physical examination revealed acneiform skin lesions on the chest, abdomen, and upper and lower limbs, which also showed subcutaneous nodules. In addition, a violaceous macula also stood out on the right lower limb (Figure 1). Pulmonary auscultation revealed diffuse rumbles. Lymph node enlargement and changes in other systems were not identified.
Figure 1. Violaceous macule of Kaposi’s Sarcoma in the distal third of the right leg (circle). Acneiform lesions on the lateral side of the right foot caused by histoplasmosis (arrow).
Blood tests showed a CD4+ T lymphocyte count of 36 cells/µl, 11,077 copies/mL HIV viral load, 12.2 g/dL hemoglobin, 7,300/mm3 leukocytes (5,400/mm3 neutrophiles, 1,200/mm3 lymphocytes), 372 mil/mm3 platelets, 1.2 mg/dL creatinine, 94 mg/dL urea, 150 mmol/L serum sodium, 350 mg/L C-reactive protein (CRP), 250 U/L lactate dehydrogenase (LDL), and 0.5 mg/dL total bilirubin. The results from the analysis of the cerebrospinal fluid (CSF) collected for the headache were normal (15 cmH2O opening pressure, 2 cells/mm3, 93% lymphocytes, 7% monocytes, 29 mg/dL CSF protein concentration, 49 mg/dL CSF glucose concentration, and negative India ink staining). Other exams did not show relevant alterations. Chest tomography revealed bronchiectasis in the upper lobes and nodules with a miliary distribution pattern (Figure 2). Computed tomography (CT) scans of the head and abdomen were normal.
Figure 2. Chest tomography showing bronchiectasis and nodules with a miliary distribution pattern.
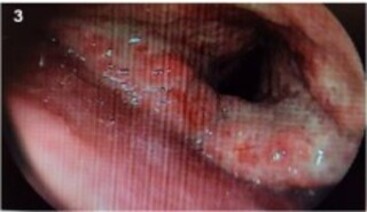
The acid-fast bacillus (AFB) test and the rapid molecular test for TB (RMT-TB) were negative in two sputum samples. On bronchoscopy, lobulated and granulomatous nodular lesions were observed in the supraglottis and aryepiglottic folds (Figure 3). The search for AFB and RMT-TB were also negative on bronchoalveolar lavage (BAL). However, laryngeal biopsies led to the identification of numerous yeasts, with a morphology suggestive of Histoplasma sp (Figure 4). These yeasts were also found in the skin biopsy of the left forearm. In addition to histology, a microbiological analysis showed Histoplasma sp growth in BAL and skin cultures after 14 days of incubation, with no fungal growth in the blood and CSF cultures. Lastly, immunodiffusion serology for histoplasmosis showed a 1:64 titer (and non-reagent serology for paracoccidioidomycosis). The polymerase chain reaction (PCR) test for blood histoplasmosis was not performed because this resource was temporarily unavailable. Due to intense odynophagia, a nasogastric tube was placed by endoscopy, which revealed a lesion suggestive of Kaposi’s sarcoma (KS). This diagnosis was confirmed by biopsy of the gastric mucosa.
Figure 3. Image of the larynx acquired during bronchoscopy showing ulcerations and edema in the supraglottis and aryepiglottic folds.
Figura 4. Histopathology of laryngeal biopsy. (A) 100X hematoxylin and eosin (H&E) staining - Fragment of the laryngeal mucosa with ulcerated granulomatous lesions showing chronic inflammation. (B) 400X Periodic acid-Schiff (PAS) staining - Cytoplasm of a multinucleated giant cell containing numerous yeasts. (C) 1000X Grocott’s methenamine silver staining - Numerous small, round, or slightly oval, single-budding yeasts measuring from 2 to 4 µm, forming clusters or rows.
Based on these results, the presumptive diagnosis of TB and its treatment, which had been restarted in this second hospitalization, were reassessed. Considering the diagnosis of disseminated histoplasmosis with cutaneous, pulmonary, and upper respiratory tract involvement, treatment with amphotericin B deoxycholate was started and subsequently replaced by amphotericin B lipid complex due to impaired renal function, adding up to a total dose of 2,500 mg. After two weeks of treatment with the antifungal agent and resumption of highly active antiretroviral therapy (HAART), the patient showed significant improvements in the skin lesions and in the dyspnea, cough, hoarseness, and odynophagia symptoms, even resuming oral feeding. He was discharged after 20 days of hospitalization for outpatient follow-up using itraconazole, HAART, and prophylaxis with trimethoprim-sulfamethoxazole and azithromycin. The treatment for cutaneous-gastric KS was performed under a day hospital regimen, achieving clinical regression of the cutaneous and gastric lesion after five chemotherapy sessions. Eight months after resuming HAART, the patient had a CD4 count of 230 cells/mL and an undetectable HIV viral load.
DISCUSSION
Histoplasmosis is highly endemic in Latin America, as shown by the percentage of positive histoplasmin skin tests (19.3%) of the general population2. Exposure to soil contaminated with bird and bat droppings and excavation or mining work in caves and caverns are directly related to inhalation of Histoplasma sp 2 microconidia. In large urban centers, construction and demolition activities have the potential to spread airborne microconidia for miles, a factor that may be related to disease outbreaks2. Moreover, exposure to Histoplasma sp does not lead to immunity to reinfection6.
The disseminated form of histoplasmosis that includes lung involvement and skin lesions1,2 is the most prevalent in Brazil, accounting for 81% of cases2, and the respiratory tract is the primary site of infection6. The main risk factor for the disease is immunosuppression, accounting for 56% of cases, while HIV infection in the AIDS phase is the most common cause (97%)2. In AIDS patients, a CD4 count lower than 150 cells/µL is the factor most commonly associated with disseminated disease5-7, with death from the disease reaching rates of 30 to 42% of cases1,2,8.
Although the reactivation of latent microorganisms that cause disseminated disease is a frequently cited mechanism, there is no evidence that reactivation is the main cause of histoplasmosis in individuals living with HIV (PLHIV)6,9, even considering the high reactivity to the histoplasmin test2. Disseminated disease may result from new exposure to and inhalation of large amounts of inocula, leading to reinfection10, 11.
Histoplasmosis manifestations can be systemic, such as fever, weight loss, lymphadenopathy, and pancytopenia. Patients with skin involvement may present with acneiform lesions in the dermis and subcutaneous nodules6,10 - characteristics found in the present case. In the respiratory system, histoplasmosis can occur with hypoxemia, dry cough, or hemoptysis, especially in acute cases; whooping cough is more common in chronic cases. In the acute form of the disease, lung imaging usually shows micronodules with a miliary distribution. By contrast, in chronic cases, larger nodules prevail, with or without cavitations6,10. Central nervous system (CNS) involvement mainly includes mental confusion and encephalopathy10. In a patient with AIDS and nonspecific symptoms, the clinical findings and examinations that should be most often considered are hepatomegaly, pancytopenia, serum lactate dehydrogenase higher than 1000 U/L, and chest image with infiltrate in a miliary pattern6,7.
The oropharynx is compromised less frequently than other organs and systems. Its compromise is usually associated with pulmonary and disseminated forms of the disease, and in a case series, a higher frequency was identified in PLHIV12,13. In this context, the physicians must consider not only laryngeal tuberculosis, but also mucosal damage by paracoccidioidomycosis and primary neoplasms as differential diagnoses12. Albeit uncommon, the oropharyngeal form of histoplasmosis can make it easier to collect material for diagnosis, as well as from the skin by lesion scraping or biopsy13.
The diagnostic methods of histoplasmosis are diverse, showing variable sensitivity and specificity7. Culture is considered the gold standard method and has a variable time for fungal growth, with a two-week minimum14. Antibody identification frequently leads to false-negative results in immunosuppressed patients with disseminated disease due to their impaired immunological response; therefore, the disease cannot be ruled out based on the absence of antibodies or the presence of low titers15. Conversely, histoplasma antigen detection in blood is one of the most recommended techniques in international consensus guidelines for disseminated disease and for treatment follow-up, as well as PCR testing for histoplasma detection in blood and BAL; more recently, the rapid urinary antigen test has increased the chances of diagnosing this disease in patients with AIDS14-18. Although samples can be easily collected and these methods show high sensitivity, they are not widely available and remain expensive. Histopathology is a fast and cost-effective method; however, yeasts may not be detected due to the limited sample amount and variable fungal load. Differential diagnoses of Paracoccidioides brasiliensis, Pneumocystis jirovecii, and Leishmania spp. must be considered because their morphological aspects are similar to those of Histoplasma sp19. Thus, physicians should combine two or more diagnostic methods to increase the chances of identifying the fungus15-18.
Due to low clinical suspicion, the diagnosis of histoplasmosis is still a challenge, often performed accidentally during the investigation of other diagnostic hypotheses, or only in the late and advanced phase of the disease6, or even only post mortem, which occurs in 18% cases of death from this disease2. The diagnosis of histoplasmosis should be considered in immunosuppressed patients with negative sputum smear microscopy or BAL, or even during follow-up of the clinical progression of patients undergoing empirical treatment or with microbiological evidence of TB but without the improvement of symptoms, not forgetting that both diseases may occur concurrently4-7,18.
The treatment depends on the severity of the disease15. In moderate-to-severe cases of disseminated histoplasmosis, amphotericin B, preferably a lipid formulation, should be used for two weeks, as in this case. In cases with neurological impairment, this period must be extended by 4 to 6 weeks15,16. Itraconazole is indicated after treatment with amphotericin B as sequential therapy in severe cases or as a treatment for mild-to-moderate cases of disseminated histoplasmosis, for a minimum period of 12 months or until meeting criteria for immune recovery in HIV patients, considering sustained 150 cells/µL or higher CD4 levels under ART for 6 months or longer10,15,16. Fluconazole is not effective in treating and controlling histoplasmosis, and its use is not indicated15. Moreover, disease recurrence is observed in 22% of cases, even with an adequate adherence to treatment8. Primary prophylaxis of histoplasmosis with 200 mg/day itraconazole may also be considered in areas of high endemicity and incidence (more than 10 cases per 100 patient-years) for patients with HIV infection and with a CD4 count lower than 150 cells/µl, maintained until immune recovery10,16.
In addition to specific treatment, ART should be started early or reintroduced in PLHIV during the treatment of histoplasmosis, after ruling out CNS involvement15. Although uncommon, Immune Reconstitution Inflammatory Syndrome may occur in patients already on ART and antifungal treatment, mainly manifesting as persistence or the worsening of histoplasmosis symptoms - these cases require testing for disease reactivation, inappropriate use of medications, or even drug interactions that result in reduced absorption. The use of corticosteroids is not yet well established, and its indication is appropriate only in severe cases, as long as concomitant antifungal use and ART are maintained10.
CONCLUSION
In this case report, clinical and radiological manifestations common to both tuberculosis and histoplasmosis favored the delay in diagnosis. However, the low clinical suspicion certainly contributed to this misdiagnosis, indicating the need to consider histoplasmosis among the differential diagnoses of opportunistic diseases in immunosuppressed AIDS patients, especially in cases with pulmonary and cutaneous involvement and with CD4 counts lower than 100 cells/µL. This report also confirms that an early diagnosis and treatment are the determining factors for the resolution of this disease, reducing progression to severe forms and death. In this case, the patient survived for more than 6 months without specific treatment for disseminated histoplasmosis and had a good response to treatment; however, the fatal evolution of cases not treated early or even of those who received adequate therapy is frequent.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Basso RP, Poester VR, Benelli JL, Stevens DA, Xavier MO. Disseminated histoplasmosis in persons with HIV/AIDS, Southern Brazil, 2010-2019. Emerg Infect Dis. 2022 Mar;28(3):721-4.
2. Almeida MA, Almeida-Silva F, Guimarães AJ, Almeida-Paes R, Zancopé-Oliveira RM. The occurrence of histoplasmosis in Brazil: a systematic review. Int J Infect Dis. 2019 Sep;86:147-56.
3. Ashraf N, Kubat RC, Poplin V, Adenis AA, Denning DW, Wright L, et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020 Oct;185(5):843-65.
4. Unis G, Severo L. Chronic pulmonary histoplasmosis mimicking tuberculosis. J Bras Pneumol. 2005 Ago;31(4):318-24. DOI: https://doi.org/10.1590/S1806-37132005000400009
5. Adenis AA, Valdes A, Cropet C, McCotter OZ, Derado G, Couppie P, et al. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: a modelling study. Lancet Infect Dis. 2018 Out;18(10):1150-9.
6. Kuate MPN, Ekeng BE, Kwizera R, Mandengue C, Bongomin F. Histoplasmosis overlapping with HIV and tuberculosis in sub-Saharan Africa: challenges and research priorities. Ther Adv Infect Dis. 2021;8:1-7.
7. Falci DR, Monteiro AA, Caurio CFB, Magalhães TCO, Xavier MO, Basso RP, et al. Histoplasmosis, an underdiagnosed disease affecting people living with HIV/AIDS in Brazil: results of a multicenter prospective cohort study using both classical mycology tests and histoplasma urine antigen detection. Open Forum Infect Dis. 2019 Abr;6(4):ofz073. DOI: https://doi.org/10.1093/ofid/ofz073
8. Damasceno LS, Ramos Junior AN, Alencar CH, Gonçalves MV, Mesquita JR, Soares AT, et al. Disseminated histoplasmosis in HIV-infected patients: determinants of relapse and mortality in a north-eastern area of Brazil. Mycoses. 2014 Jul;57(7):406-13.
9. Adenis AA, Aznar C, Couppié P. Histoplasmosis in HIV-infected patients: a review of new developments and remaining gaps. Curr Trop Med Rep. 2014;1(2):119-28.
10. Myint T, Leedy N, Cari EV, Wheat LJ. HIV-associated histoplasmosis: current perspectives. HIV AIDS (Auckl). 2020;12:113-25.
11. Hanf M, Adenis A, Couppie P, Carme B, Nacher M. HIV-associated histoplasmosis in French Guiana: recent infection or reactivation? AIDS. 2010 Jul;24(11):1777-8.
12. Ferreira OG, Cardoso SV, Borges AS, Ferreira MS, Loyola AM. Oral histoplasmosis in Brazil. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002 Jun;93(6):654-9.
13. Antonello VS, Zaltron VF, Vial M, Oliveira FM, Severo LC. Oropharyngeal histoplasmosis: report of eleven cases and review of the literature. Rev Soc Bras Med Trop. 2011;44(1):26-9.
14. Guimarães AJ, Nosanchuk JD, Zancopé-Oliveira RM. Diagnosis of histoplasmosis. Braz J Microbiol. 2006 Jan;37(1):1-13.
15. Perez F, Caceres DH, Ford N, Ravasi G, Gomez BL, Pasqualotto AC, et al. Summary of Guidelines for Managing Histoplasmosis among People Living with HIV. J Fungi (Basel). 2021 Fev;7(2):134-42.
16. Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007 Out;45(7):807-25.
17. Vidal JE, Werlang PC, Muniz BM, Rego CM, Barbalho RE, Baptista AM, et al. Combining urine antigen and blood polymerase chain reaction for the diagnosis of disseminated histoplasmosis in hospitalized patients with advanced HIV disease. Med Mycol. 2021 Set;59(9):916-22.
18. Falci D, Lana DFD, Pasqualotto AC. The era of histoplasmosis in Brazilian endemic mycoses. Lancet Regional Health Am. 2021;3:100037. DOI: https://doi.org/10.1016/j.lana.2021.100037
19. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011 Abr;24(2):247-80.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















