Infectious endocarditis caused by Haemophilus parainfluenza
DOI:
https://doi.org/10.5935/2764-734X.e20240236Keywords:
Endocarditis, Bacterial, Haemophilus Infections, Haemophilus parainfluenzae, Case ReportsAbstract
Infective endocarditis caused by bacteria from the HACEK group - Haemophilus spp. (excluding Haemophilus influenza), Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae - is rare; affected patients are usually young and present with predisposing factors, such as heart disease. The infection is characterized by an insidious clinical course of subacute presentation, with an average delay in diagnosis of one to three months, which may be related to the formation of larger valve vegetations and a consequent increased risk of embolization. This report describes a case of a young, previously healthy girl whose primary clinical complaint was headache and fever. The diagnostic suspicion arose after Haemophilus parainfluenza positive blood culture and was confirmed by the echocardiographic visualization of a vegetation adhering to the posterior leaflet of the mitral valve. There was a good clinical response to treatment with ceftriaxone for six weeks; however, after three months, surgery for valve replacement was necessary.
Downloads
INTRODUCTION
Infective endocarditis (IE) caused by bacteria from the HACEK group (an acronym formed by the initials of the genera Haemophilus spp., excluding Haemophilus influenzae; Aggregatibacter spp.; Cardiobacterium spp.; Eikenella spp. and Kingella spp.) is rare, accounting for 1%-3% of all cases 1. They are fastidious gram-negative bacteria, grouped together didactically due to common characteristics, such as their presence in the pharyngeal microbiota, low virulence, and ability to cause clinically similar infections, including IE. They are characterized by an insidious clinical course with subacute presentation, leading to an average delay in diagnosis of one to three months 1. Patients with IE due to HACEK are younger, usually have predisposing factors of heart disease, often have poor dentition, while the most common affected sites are the aortic (30%-49%) and mitral (45%-50%) valves 1,2.
Here, we present IE caused by a HACEK bacterium with its typical epidemiology, presentation, and progression, emphasizing that neurological manifestations can be among the first clinical signs of the disease, which in turn also causes diagnostic confusion and therapeutic delay.
CASE REPORT
This is a case of a 15-years old woman, brown-skinned, born in the western region of Rio Grande do Norte (urban area), living in São Paulo city (State of São Paulo) for about a month. She lives with her father, stepmother, and younger brother, and is in her first year of secondary school. She was admitted to the Emergency Room (ER) of a University Hospital for unilateral but migrating headache (between both sides of the head) that started eight days prior. The headache was progressive in nature with intensity 7 (seven) on the Visual Pain Scale, associated with nausea and vomiting. She reported daily febrile peaks of 38.6°C, sweating, and tremors five days before hospitalization. Previously healthy, she denied the use of continuous medication, alcoholism, smoking, or substance abuse. Additionally, she reported having received a full vaccination schedule, denied previous illnesses or hospitalizations, and reported being in follow-up by a psychologist for a depressive episode.
On physical examination at admission, she was in good general health, discolored +/4, acyanotic, sleepy, eupneic (peripheral saturation 97% on room air), with blood pressure of 100/60 mmHg and a heart rate of 112 bpm. She had pain and cervical rigidity when flexing the head, but with a negative Brudzinsky sign. Pupils were isochoric and photoreagent. Strength and sensitivity were preserved in both limbs; cardiac auscultation revealed normal heart sounds in two clicks and no murmurs; pulmonary auscultation and physical examination of the abdomen, extremities, skin, and pharynx found no relevant alterations. Peripheral blood cultures and other laboratory tests were conducted with the following results: Hb: 10.3 g/dl; Ht: 29%; leukocytes: 10,460 mm2; platelets: 105,000 ml/mm3; serum sodium: 128 mEq/l; potassium: 4.2 mEq/l; creatinine: 0.65mg/dl; urea: 19 mg/dl; and international normalized ratio of 1.32. Moreover, serology for human immunodeficiency virus infection and the reverse transcription polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 were negative.
The skull compute tomography (CT) scan showed no expansive lesions, hemorrhages, or acute findings in the brain parenchyma, allowing safe lumbar puncture for cerebrospinal fluid (CSF) collection. Analysis of the CSF revealed a clear and colorless appearance, cellularity of 1/mm3, proteins 14 mg/dl, glucose 61 mg/dl, and lactate 15mg/dl; Gram staining, cultures, and India ink staining were negative.
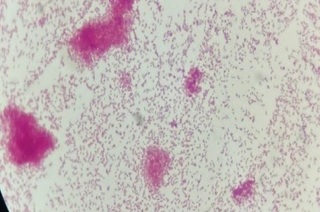
Due to the hypothesis of symptomatic hyponatremia, therapy with 3% sodium chloride (NaCl) was initially instituted. The patient remained under observation in the ER for two days, maintaining fever and tachycardia, with pain appearing on palpation in the right flank. A type 1 urine test and normal ultrasound of the kidneys and urinary tract ruled out pyelonephritis; however, it was decided to introduce antibiotic therapy with ceftriaxone 2g/day. On day 3, the patient’s drowsiness and nausea improved, and, on the same day, the microbiology laboratory informed about the growth of gram-negative cocobacilli in the blood culture (29 hours after collection), subsequently identified as multisensitive Haemophilus parainfluenzae via an automated method (Figure 1).
Figure 1. Gram-negative cocobacilli identified in the blood culture of peripheral blood.
Based on this result, a Transthoracic Echocardiogram was requested, which showed the mitral valve with prolapse of both cusps, as well as a 17 × 9 mm mobile echogenic image adhered to the valve’s posterior leaflet, generating mild-moderate reflux. This finding was compatible with an IE vegetation and was confirmed by a transesophageal echocardiogram performed 10 days after initiating the ceftriaxone treatment. After confirming the diagnosis of IE, the medical team recollected the patient’s medical history and discovered that she had undergone tooth extraction for dental caries three months before the onset of symptoms (Figure 2).
Figure 2. A friable area with necrotic tissue observed in the region of the previously extracted lower right 1st molar.
New blood samples were collected daily for three consecutive days after the initial collection. These cultures showed no bacterial growth from the first day of antibiotic therapy, which was maintained for six weeks. During this period, the patient was transferred to a cardiology specialty department for possible surgical intervention, which, initially, was not indicated as the vegetation was on the posterior leaflet of the mitral valve. Anticoagulation treatment for embolic prevention was also not indicated. Three months after discharge, however, in the follow-up, the patient presented with dyspnea, and a new echocardiogram showed significant mitral valve regurgitation. Consequently, she underwent valve replacement surgery. According to the medical records, there were no complications and the patient showed marked clinical improvement in the immediate post-operative period, being discharged asymptomatic for long-term outpatient follow-up.
DISCUSSION
This report corresponds to the most common characteristics of IE caused by the HACEK group (young age, insidious clinical course, larger vegetations) and also brings a frequent triggering factor, although only revealed late: the dental procedure.
Headache was the primary symptom presented by the patient and, as such, became a confounding factor in the formulation of diagnostic hypotheses. Neurological manifestations, however, are known to be the first clinical sign of an IE, including other isolated symptoms like seizures or trigeminal neuralgia 3. Another neurological complication of IE is meningitis, reported in approximately 3.5% of patients. Typically, an aseptic pattern is seen in the CSF, with mild mononuclear pleocytosis. This aseptic pattern may be attributed to parameningeal inflammation, pretreatment with antibiotics, or even a low bacterial load in CSF 3. Other phenomena directly or indirectly related to IE include transient ischemic episodes, intracerebral or subarachnoid hemorrhage, toxic encephalopathy, and cerebral abscesses, with clinically asymptomatic cerebral embolisms occurring in 35%-60% of these patients 4.
Blood culture is the most important test during the initial propaedeutics of IE 4,5; at least three pairs of blood cultures are recommended before initiating antibiotic therapy 4,6. According to the revision of the “Duke criteria,” published in 2023 6, bacteria from the HACEK group are classified as “typical” in IE because their growth in blood cultures is strongly associated with endocarditis, a fact that gives them the status of a “major” criterion when isolated in two or more sets of the samples 6. Moreover, as they are rarely isolated in blood cultures from patients without endocarditis, there is an argument stating that a single positive blood culture may be already sufficient to validate IE diagnosis 7. In a study carried out in New Zealand, 87 cases of HACEK bacteremia were identified from electronic databases. The overall positive predictive value of HACEK bacteremia for the diagnosis of endocarditis was 60%, varying according to species: from 0% (E. corrodens) to 100% (A. actinomycetemcomitans) 7. The insidious presentation of the disease with consequent diagnostic and therapeutic delays is the main evidence for patients with HACEK IEs developing large vegetations with high probability of embolization 1.
The treatment of choice is a third-generation cephalosporin (ceftriaxone), leading to a favorable outcome in up to 90% of cases 4. In 40%-70% of cases, however, cardiac surgery is necessary, with a mortality rate of 5%-10% 3,8. The duration of therapy, calculated from the day on which blood cultures are negative, must be sufficient to completely eradicate the microorganism within the vegetations. Therefore, blood cultures should be taken every 24-72 hours until demonstrated elimination of the bloodstream infection 5. Both the American Heart Association (AHA) and the European Society of Cardiology (ESC) recommend maintaining cephalosporin for four weeks after the first negative blood culture, in native valves 4,9.
The embolic risk associated with IE by the HACEK group is very high, especially during the first two weeks 4, with events occurring in 20%-50% of patients. Patients with valve vegetations >10 mm in diameter have a higher risk of embolism, and this risk is even higher in patients with mobile vegetations >15 mm 10. Early cardiac surgery in high-risk patients is essential to prevent embolization of vegetations 4. In contrast, antithrombotic or thrombolytic medical therapies have not proved beneficial 9,10 and are only used in cases of left-sided IE in patients already under previous anticoagulation therapy.
In general, the indications for surgery in patients with IE are related to heart failure or shock, evidence and risk of persistent infection, and reducing embolic risk 4. It is important to note that the AHA and ESC guidelines differ in terms of the indications for surgery to prevent embolism. In our patient, for example, according to the recommendations of the AHA 9, there is no recommendation for an early surgical procedure, as the indication based solely on the size of the vegetation (>10 mm) is limited to involvement of the anterior portion of the mitral or aortic valve. Conversly, ESC 4 considers the possibility of surgery whenever the size of any vegetation is >10 mm, regardless of its location. These differences reinforce the need for a multidisciplinary discussion in order to decide on a more individualized therapy, with consensual assessment on a case-by-case basis.
CONCLUSION
This case report and the brief accompanying literature discussion highlight the type of patient and clinical course of IE caused by bacteria of the HACEK group, which usually responds well to clinical treatment. Frequent valve vegetations, however, represent increased risk of structural complications in the short and medium terms and may cause delays in diagnosis - in such cases, valve replacement surgeries are not uncommon. Controlled clinical trials and robust meta-analyses are still lacking to assess the benefits of prophylactic antiaggregants and anticoagulants in order to reduce embolic risk, while antibiotic therapy and surgery, when indicated, remain essential for embolic prevention and cure of IE.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1932-5
2. Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almela M, et al. HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One. 2013;8(5):e63181. DOI: 10.1371/journal.pone.0063181
3. Ferro JM, Fonseca AC. Infective endocarditis. Handb Clin Neurol. 2014;119:75-91. DOI: 10.1016/B978-0-7020-4086-3.00007-2
4. Delgado V, Marsan NA, Waha S, Bonaros N, Brida M, Burri H, et al. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J. 2023;44(39):3948-4042. DOI: 10.1093/eurheartj/ehad193
5. Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler Junior VG. Infective endocarditis. Nat Rev Dis Primers. 2016;2:16059. DOI: 10.1038/nrdp.2016.59
6. Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin Infect Dis. 2023;77(4):518-26. DOI: 10.1093/cid/ciad271
7. Yew HS, Chambers ST, Roberts SA, Holland DJ, Julian KA, Raymond NJ, et al. Association between HACEK bacteraemia and endocarditis. J Med Microbiol. 2014;63(Pt 6):892-5. DOI: 10.1099/jmm.0.070060-0
8. Gagliardi R, Sensi C, Flaminio G, De Canale E, Vettor R, De Carlo E. Haemophilus parainfluenzae endocarditis in a low-risk woman: a case report. Clin Case Rep. 2021;9(11):e05066. DOI: 10.1002/ccr3.5066
9. Baddour LM, Wilson WR, Bayer AS, Fowler Junior VG, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015;132:1435-86. DOI: 10.1161/CIR.0000000000000296
10. Mohananey D, Mohadjer A, Pettersson G, Navia J, Gordon S, Shrestha N, et al. Association of vegetation size with embolic risk in patients with infective endocarditis: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(4):502-10. DOI: 10.1001/jamainternmed.2017.8653
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















