Typhoid fever as a cause of acute abdomen: a differential diagnosis to remember
DOI:
https://doi.org/10.5935/2764-734X.e20240337Keywords:
Typhoid Fever, Salmonella typhi, Intestinal Perforation, Tuberculosis, Gastrointestinal, Case ReportsAbstract
Infection caused by Salmonella typhi continues to be a major global health concern, especially in regions with limited access to basic sanitation. Typhoid fever is characterized by a wide variety of clinical manifestations and aggressiveness in causing necrosis of the tissue. It is difficult to diagnose in endemic areas where other diseases with similar symptoms are more common, which increases the potential morbidity and mortality. In this article, we report the case of a patient with typhoid fever who was initially wrongly diagnosed with intestinal tuberculosis and whose condition progressed to perforation and death.
Downloads
INTRODUCTION
Although worldwide efforts have been made to eliminate typhoid fever, it remains a significant global health problem, especially in developing countries and areas with limited access to sanitation1,2. Despite being endemic in several regions, this disease is significantly under-reported and given insufficient recognition in the differential diagnosis of abdominal pathologies3,4.
Typhoid fever has a nonspecific clinical spectrum, including fever, abdominal pain, diarrhoea alternating with constipation, skin rash, asthenia, prostration, headache, and encephalopathy, which further contributes to delayed diagnosis and initiation of appropriate treatment. As a consequence, the risk of disease-related complications and death increases5.
We report a case of typhoid fever complicated by intestinal perforation, a condition that poses a diagnostic challenge with other causes of acute abdominal inflammation, such as suppurative appendicitis and colitis, and is associated with high morbidity and mortality.
CASE REPORT
A 34-year-old homeless man presented to the emergency department of a tertiary hospital with complaints that had started 5 days before admission. He experienced diarrhea without pathological products accompanied by general malaise, nausea, vomiting of food contents, and intense, diffuse, cramp-like abdominal pain, which led him to seek medical help. He did not have a history of abdominal trauma, physical aggression or previous surgery, but he had a history of use of psychoactive substances (crack and alcohol). On physical examination, he was dehydrated, febrile (axillary temperature of 38.3 °C), normotensive, and normocardic (heart rate of 65 beats per minute) and dehydrated. He was eupneic in room air, and cardiopulmonary auscultation showed no significant changes. The abdomen was distended and diffusely tender on superficial and deep palpation, with a guarding sign. There were no skin lesions, lymphadenopathy, or changes in other systems.
Initial laboratory tests showed leukopenia with neutropenia and lymphopenia, an increase in inflammatory markers, an increase in liver, pancreatic, and canalicular enzymes, hyperbilirubinemia at the expense of direct bilirubin, and hyponatremia (Table 1). Antibody tests for human immunodeficiency virus (HIV) 1 and 2 were negative, as were serological tests for hepatitis A, B, and C. FTA-ABS treponemal test was reactive, while VDRL non-treponemal test showed a titer of 1:2. The other tests did not show any relevant changes. Peripheral blood samples were also obtained and inoculated in culture media for aerobic and anaerobic bacteria.
| Laboratory test | Results | Reference values |
|---|---|---|
| Hemoglobin | 14.4 g/dL | 13.0-18.0 g/dL |
| Leukocytes | 2,300 /mm3 | 4,000-11,000 /mm3 |
| Neutrophils | 1500 /mm3 | 1,600-7,000 /mm3 |
| Lymphocytes | 400 /mm3 | 900-3,400 /mm3 |
| Platelets | 117,000 /mm3 | 140,000-450,000 /mm3 |
| Urea | 25 mg/dL | 19-43 mg/dL |
| Creatinine | 0.5 mg/dL | 0.66-1.25 mg/dL |
| Sodium | 118 mmol/L | 137-145 mmol/L |
| C-reactive protein | 374.5 mg/L | < 5.0 mg/dL |
| Lactic dehydrogenase | 733 U/L | 120-246 U/L |
| Alanine aminotransferase | 110 U/L | < 50 U/L |
| Aspartate aminotransferase | 492 U/L | < 59 U/L |
| Alkaline phosphatase | 142 U/L | 38-126 U/L |
| Gamma glutamyltransferase | 144 U/L | 15-73 U/L |
| Amylase | 131 U/L | 30-110 U/L |
| Lipase | 654 U/L | 25-300 U/L |
| Total bilirubin | 1.4 mg/dL | 0.2-1.3 mg/dL |
| Direct bilirubin | 1.1 mg/dL | 0.1-0.5 mg/dL |
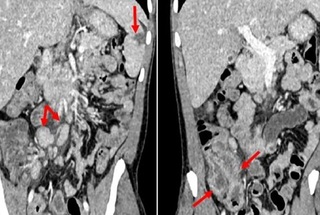
Abdominal computed tomography (CT) revealed mild hepatosplenomegaly, a peripheral hypoattenuating lesion in the spleen (suggestive of an area of localized infarction), signs of terminal ileitis and typhlitis associated with regional and retroperitoneal lymph node enlargement (Figure 1).
Figure 1. Abdominal computed tomography scans in coronal reconstructions of the portal phase showing (A): discrete hepatosplenomegaly with signs of fatty infiltration of the liver, small area of peripheral infarction in the splenic parenchyma (larger arrow) and retroperitoneal and mesenteric lymph node enlargement (smaller arrows); in (B), signs of parietal thickening and enhancement of the terminal ileum and cecum indicating ileitis and typhlitis (arrows), with slight blurring of regional mesenteric fat planes.
Faced with the suspicion of acute abdominal inflammation, the initial surgical approach was conservative. Therefore, antibiotic therapy for abdominal infection with ceftriaxone and metronidazole, parenteral hydration, analgesia, and clinical surveillance was initiated. On the second day of hospitalization, the patient had severe enterorrhagia with hemodynamic instability and was transferred to the intensive care unit (ICU) where vasoactive drugs were given. When the circulatory shock subsided, colonoscopy was performed on the third day of hospitalization, which showed ulcerated lesions in the colon and ulcerative ileitis with areas of necrosis, indicative of tuberculous mycobacteriosis (Figure 2). Given this hypothesis, an alternative intravenous regimen was started for the treatment of tuberculosis with levofloxacin, linezolid, and amikacin (metronidazole was also maintained to cover anaerobic bacteria), assuming that the absorption of any medication in the gastrointestinal tract was compromised due to significant local involvement. The patient did not develop fever or new episodes of gastrointestinal bleeding, but began to have intestinal constipation and increased abdominal distension in the following days.
Figure 2. Colonoscopy images. (A) and (B): clots and deep ulcerations with fibrin in the swollen distal ileum (arrows). (C) and (D): irregular ulcerated lesions measuring around 2.5-3.0 cm in diameter, slightly granular, with well-defined edges and centers with hyperemia points located in the hepatic angle (arrows).
After 7 days in the hsssospital, blood cultures collected on admission showed the growth of Salmonella enterica serotype typhi, the susceptibility profile of which is shown in Table 2. Based on this confirmed diagnosis of typhoid fever, ceftriaxone was reinitiated, and treatment for tuberculosis was suspended. The colonoscopy biopsies, however, showed chronic and erosive ileitis and colitis with histiocytic aggregates outlining granulomas (Figure 3), which again raised the possibility of tuberculosis. Although Ziehl-Neelsen staining was negative for acid-fast bacilli, the tuberculosis treatment regimen was reintroduced and maintained for a few more days. It was only after an immunohistochemical analysis of BCG antigens carried out on the same biopsies that it was decided definitively to discontinue the treatment.
| Antibiotics | Minimum InhibitoryConcentration (µg/mL) | Interpretation |
|---|---|---|
| Amikacin | ≤ 8 | Sensitive |
| Ampicillin | ≤ 4 | Sensitive |
| Ampicillin-Sulbactam | ≤ 4/2 | Sensitive |
| Cefepime | ≤ 1 | Sensitive |
| Ceftazidime | ≤ 1 | Sensitive |
| Ceftriaxone | ≤ 1 | Sensitive |
| Ertapenem | ≤ 0.25 | Sensitive |
| Gentamicin | ≤ 2 | Sensitive |
| Imipenem | ≤ 0.25 | Sensitive |
| Meropenem | ≤ 0.5 | Sensitive |
| Piperacillin-Tazobactam | ≤ 4/4 | Sensitive |
| Sulfamethoxazole-Trimethoprim | ≤ 0.5/9.5 | Sensitive |
Figure 3. (A): anatomical specimen showing a segment of small intestine with hemorrhagic mucosa and the presence of elliptical ulcers on Peyer’s patches (arrows). (B): histological section showing moderate, erosive chronic ileitis, with a histiocytic aggregate outlining a granuloma (arrow with semicircle), exudative foci and granulation tissue in the lamina propria (arrows) [H.E., 400x magnification]; in (C) and (D); deep ulcerated lesions extending to the tunica muscularis (arrows) [H.E., 100x magnification and H.E. 200x magnification, respectively]. H.E. = hematoxylin and eosin stain.
Pneumoperitoneum was observed on a plain abdominal X-ray on day 11 of hospitalization. The surgical team performed emergency exploratory laparotomy, and the findings were as follows: peritonitis with more than two liters of pus and enteric fluid with fibrin throughout the abdominal cavity; pelvic abscess with one liter more of pus and fibrin; encapsulating peritonitis with firm adherence of the right colon, without a cleavage plane with the right paracolic gutter, as well as loose adhesions between the small bowel loops. Three areas of perforation were observed in the terminal ileum with everted edges and 2 cm in diameter each, approximately 4 cm apart. After segmental enterectomy with terminal ileostomy and washing of the abdominal cavity, the surgical specimen was sent for pathological analysis (Figure 3), which showed a segment of the small intestine with chronic, active, transmural, ulcerated and perforated inflammation, as well as acute purulent serositis with granulomas and a foreign body-type giant cell reaction involving food remains. In addition, samples of intra-abdominal pus were collected for microbiological analysis and the results of the acid-fast bacilli test, mycobacteria culture, and rapid molecular assay for M. tuberculosis were all negative, as was the search for and culture of fungi. In the cultures for aerobic bacteria, the following were isolated: Enterobacter cloacae, Enterococcus faecium, and Acinetobacter baumannii - the respective sensitivity profiles are listed in Table 3. The antimicrobial regimen was thus extended to include vancomycin, amikacin, imipenem, and polymyxin. Despite the treatment, the patient showed a significant drop in hematocrit levels on the 18th day of hospitalization and a second abdominal CT scan showed the presence of multiple intra-abdominal collections. A second exploratory laparotomy showed moderate hemoperitoneum, persistent inflammatory enteritis, and partial dehiscence of the aponeurosis with eventration. The patient was kept under mechanical ventilation, and his clinical condition progressively deteriorated. Hemodynamic instability refractory to vasoactive drugs and multiple organ dysfunction culminated in death on day 35.
| Antibiotics | Isolated bacteria | |||
|---|---|---|---|---|
| Acinetobacter baumannii * | Enterobacter | Enterococcus faecium * | ||
| MIC | Enterobacter cloacae | |||
| Amikacin | NT | > 16 | Resistant | Resistant |
| Ampicillin | NT | >16 | Resistant | Resistant |
| Ampicillin-Sulbactam | Resistant | >16/8 | Resistant | NT |
| Cefepime | NT | >16 | Resistant | NT |
| Ceftazidima | NT | >16 | Resistant | NT |
| Ceftriaxone | NT | >4 | Resistant | NT |
| Ciprofloxacin | Resistant | >2 | Resistant | NT |
| Ertapenem | NT | >1 | Resistant | NT |
| Streptomycin (high levels) | NT | NT | NT | Resistant |
| Gentamicin | Resistant | >8 | Resistant | NT |
| Gentamicin (high levels) | NT | NT | NT | Sensitive |
| Imipenem | Resistant | 4 | Intermediate | NT |
| Levofloxacin | Resistant | >4 | Resistant | NT |
| Linezolid | NT | NT | NT | Sensitive |
| Meropenem | Resistant | 16 | Resistant | NT |
| Piperacillin-Tazobactam | NT | 64/4 | Resistant | NT |
| Tigecycline | Resistant | NT | NT | NT |
| Vancomycin | NT | NT | NT | Sensitive |
| Polymyxin B | NT | 0,25 | Sensitive | NT |
DISCUSSION
Typhoid fever is an infectious disease caused by Salmonella enterica serotype typhi, a gram-negative, non-spore-forming, facultative anaerobic bacillus, which is transmitted by the oral-fecal route, usually through drinking water and consumption of foods contaminated with the microorganism. There is no animal reservoir other than humans in which the bacterium causes a clinical disease after incubation for one to three weeks. Recovered individuals can continue to excrete bacilli into the environment, thereby continuing the chain of transmission by contaminating water and food sources in the absence of proper sanitation. Furthermore, direct contact with sick individuals or carriers is another possible mode of transmission5,6.
In 2019, approximately 9 million people in the world were affected by the disease4, causing around 110,000 deaths. These figures place typhoid fever as an important cause of death from infectious diseases at a high global position1. In 2019, only 69 cases were confirmed in Brazil, with 12 cases occurring in the Southeast, eight of which were in the state of Sao Paulo7,8. Despite mandatory notification, there is most likely underdiagnosis or underreporting. Approximately 10%-15% of patients with typhoid fever develop enterorrhagia and intestinal perforation (mainly ileal), which directly compromise the prognosis9. In the pre-antimicrobial era, the mortality rate was as high as 30%, but today it is less than 1%2. However, it is essential to consider this disease in the differential diagnosis of acute febrile illnesses with acute abdomen, especially in endemic regions.
The culture of clinical specimens is the main diagnostic method because it enables the isolation and identification of Salmonella enterica species and its antimicrobial susceptibility profile. Blood cultures, especially during the first and second weeks of the disease, have a sensitivity of at least 80% when performed before antibiotic treatment10. Conversely, myeloculture is highly sensitive even during antibiotic therapy. In the second and third weeks, stool tests may be carried out, but sensitivity varies between 10% and 30% depending on the number of samples taken10. The serological test using the Widal reaction, which measures agglutinating antibodies against antigens in Salmonella typhi structure, is a simple and inexpensive method that is still used in many regions as a diagnostic aid; however it demands careful interpretation of the results10.
Colonoscopy is an important diagnostic and therapeutic method for detecting and managing hemostasis in cases of gastrointestinal bleeding. Endoscopic findings, such as ulcers of varying sizes and shapes, predominantly located in the terminal ileum, appendix, and right colon, are quite frequent but not specific, and often raise higher suspicion of other diseases such as intestinal tuberculosis, malignancy, and Chron’s disease11. Intestinal wall thickening with lymphoid tissue infiltration and raised nodules corresponding to hyperplasia of Peyer’s patches are histologically observed. Linear or discoid ulcers progress to transmural necrosis, which culminates in intestinal perforation. Intestinal granulomas are rare in patients with typhoid fever but are occasionally found12: infiltrates rich in histiocytes mixed with lymphocytes and plasma cells with areas of central non-caseating necrosis should favor suspicion13.
It is important to note that the clinical and radiological similarities between typhoid fever and intestinal tuberculosis often lead to a diagnostic reasoning in favor of the latter because tuberculosis is the most reported specific infectious cause of acute nontraumatic bowel perforation in countries with a high epidemiological burden, such as Brazil14,15. Obtaining clinical samples for testing with targeted staining and culture media is therefore essential 13. However, unlike mycobacteria16, the recovery of Salmonella typhi in these materials is usually unsatisfactory. Techniques such as immunohistochemistry are used to test for monoclonal antibodies against the O:9 antigen and the virulence factor (Vi) of Salmonella spp. However, these methods require more advanced technological equipment that is not available in all facilities17.
Treatment of typhoid fever is based on antibiotic therapy and the management of complications. Ampicillin, chloramphenicol, and cotrimoxazole have been used as first-line antibiotics in this treatment for many years10. Resistance to these drugs, however, has already been considered endemic in some Asian and African countries, so ciprofloxacin, azithromycin, and ceftriaxone are the next option. However, the widespread use of ciprofloxacin has already caused its resistance to spread and it is no longer an ideal choice10. Azithromycin is still considered an effective oral alternative for uncomplicated cases, while ceftriaxone is the best option for hospitalized patients [although there are records of resistance in Pakistan18], especially when there is uncertainty about the absorption of medication in the gastrointestinal tract, as in the present case. The fact is that the spread of multidrug-resistant strains of Salmonella typhi in several regions of the world contributes to therapeutic failure and prolonged elimination of the microorganism in feces. The surgical approach in cases of intestinal perforation is obviously essential, but it is still challenging for health services in developing countries, where patients usually present late and with advanced disease and there is often a lack of adequate diagnostic and treatment resources19.
CONCLUSION
The present case illustrates the importance of including typhoid fever as an etiological diagnosis of acute abdominal inflammation, especially in individuals who live in precarious conditions in terms of hygiene and access to basic sanitation. It is also important to highlight the non-specificity of the signs and symptoms and the overlap of clinical presentations with intestinal tuberculosis, particularly in areas endemic for both diseases, such as Brazil. Rapid clinical deterioration is a serious complication that requires early diagnosis and immediate intervention. It may be associated with gastrointestinal bleeding, intestinal perforation and resistance of bacteria to prescribed antibiotics.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Chen J, Long JE, Vannice K, Shewchuk T, Kumar S, Steele AD, et al. Taking on Typhoid: eliminating typhoid fever as a global health problem. Open Forum Infect Dis. 2023;10(Suppl 1):S74-S81. DOI: 10.1093/ofid/ofad055
2. Crump JA. Progress in typhoid fever epidemiology. Clin Infect Dis. 2019;68(Suppl 1):S4-S9. DOI: 10.1093/cid/ciy846
3. Marchello CS, Hong CY, Crump JA. Global typhoid fever incidence: a systematic review and meta-analysis. Clin Infect Dis. 2019;68(Suppl 2):S105-S116. DOI: 10.1093/cid/ciy1094
4. Hancuh M, Walldorf J, Minta AA, Tevi-Benissan C, Christian KA, Nedelec Y, et al. Typhoid fever surveillance, incidence estimates, and progress toward typhoid conjugate vaccine introduction - worldwide, 2018-2022. Morb Mortal Wkly Rep. 2023;72(7):171-6. DOI: 10.15585/mmwr.mm7207a2
5. Focaccia R, Mattei SMM, Saravía-Gomez J, Lima VP. Febres tifoide e paratifoide. In: Veronesi R, Focaccia R, eds. Tratado de Infectologia: v.1. 5a Edição Revisada e Atualizada. São Paulo: Editora Atheneu; 2015. p. 1163-75.
6. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual Integrado de vigilância e controle da febre tifoide [Internet]. Brasília: Editora MS; 2010; [access in 2023 Oct 01]. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/manual_integrado_vigilancia_febre_tifoide.pdf
7. Azevedo CP, Paes ALV, Duarte AS, Azevedo AL, Lima GES, Parente FA, et al. Analysis of the epidemiological profile of typhoid and paratyphoid fever in Brazil from 2014 to 2018. Braz J Hea Rev. 2020 Jul;3(4):8789-92. DOI: 10.34119/bjhrv3n4-124
8. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Sistema de Informação de Agravos de Notificação - Sinan [Internet]. Brasília; 2019; [access in 2023 Oct 01]. Available from: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/febretifoidebr.def
9. Khanam F, Ross AG, McMillan NAJ, Qadri F. Toward typhoid fever elimination. Int J Infect Dis. 2022;119:41-3. DOI: 10.1016/j.ijid.2022.03.036
10. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev. 2015;28(4):901-37. DOI: 10.1128/CMR.00002-15
11. Lee JH, Kim JJ, Jung JH, Lee SY, Bae MH, Kim YH, et al. Colonoscopic manifestations of typhoid fever with lower gastrointestinal bleeding. Dig Liver Dis. 2004;36(2):141-6. DOI: 10.1016/j.dld.2003.10.013
12. Cheung C, Merkeley H, Srigley JA, Salh B, Webber D, Voyer S. Ileocecal ulceration and granulomatous ileitis as an unusual presentation of typhoid fever. CMAJ. 2012;184(16):1808-10. DOI: 10.1503/cmaj.120714
13. Amarnath S, Deeb L, Philipose J, Zheng X, Gumaste V. A comprehensive review of infectious granulomatous diseases of the gastrointestinal tract. Gastroenterol Res Pract. 2021;2021:8167149. DOI: 10.1155/2021/8167149
14. Gomes T, Reis-Santos B, Bertolde A, Johnson JL, Riley LW, Maciel EL. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect Dis. 2014;14:9. DOI: 10.1186/1471-2334-14-9
15. Rocha EL, Pedrassa BC, Bormann RL, Kierszenbaum ML, Torres LR, D’Ippolito G. Abdominal tuberculosis: a radiological review with emphasis on computed tomography and magnetic resonance imaging findings. Radiol Bras. 2015;48(3):181-91. DOI: 10.1590/0100-3984.2013.1801
16. Birkhold M, Datta S, Pak GD, Im J, Ogundoyin OO, Olulana DI, et al. Characterization of typhoid intestinal perforation in africa: results from the severe typhoid fever surveillance in africa program. Open Forum Infect Dis. 2023;10(Suppl 1):S67-S73. DOI: 10.1093/ofid/ofad138
17. Neil KP, Sodha SV, Lukwago L, O-tipo S, Mikoleit M, Simington SD, et al. A large outbreak of typhoid fever associated with a high rate of intestinal perforation in Kasese District, Uganda, 2008–2009. Clin Infect Dis. 2012;54(8):1091-9. DOI: 10.1093/cid/cis025
18. Masuet-Aumatell C, Atouguia J. Typhoid fever infection - antibiotic resistance and vaccination strategies: a narrative review. Travel Med Infect Dis. 2021;40:101946. DOI: 10.1016/j.tmaid.2020.101946
19. Nguyen QC, Everest P, Tran TK, House D, Murch S, Parry C, et al. A clinical, microbiological, and pathological study of intestinal perforation associated with typhoid fever. Clin Infect Dis. 2004;39(1):61-7. DOI: 10.1086/421555
20. Ministério da Saúde (Brasil). Secretaria de Vigilância em Saúde. Portaria nº 64 de 11 de dezembro de 2018. Determina aos laboratórios da rede pública e rede privada, de todas as Unidades Federadas, a utilização das normas de interpretação para os testes de sensibilidade aos antimicrobianos (TSA), tendo como base os documentos da versão brasileira do European Committee on Antimicrobial Susceptibility Testing [Internet]. Brasília: Ministério da Saúde; 2018; [access in 2023 Dec 23]. Available from: https://bvs.saude.gov.br/bvs/saudelegis/svs/2018/prt0064_14_12_2018.html
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















