Mycobacterium abscessus infection after aesthetic surgical procedure
DOI:
https://doi.org/10.5935/2764-734X.e20231030Keywords:
Mycobacterium abscessus, Mycobacterium Infections, Nontuberculous, Postoperative Complications, Plastic Surgery Procedures, Case ReportAbstract
Infections caused by Mycobacterium abscessus are uncommon, however, cases are emerging, especially associated with trauma and infections related to health care after aesthetic surgical procedures. The diagnosis is difficult and the treatment is long time. Although mortality rates are low, it is associated with significant morbidity, The authors describe a case of Mycobacterium abscessus infection with myocutaneous manifestation, after abdominal liposuction and gluteal graft with diffuse ulcer-crusted lesions, subcutaneous and myofascial collections, fasciitis and constitutional symptoms. The diagnosis was obtained by culture of secretion. The treatment was started with amikacin, tigecycline, clarithromycin and ertapenem and the maintenance with amikacin, clarithromycin and clofazimine, with regresson of the lesions.
Downloads
INTRODUCTION
Mycobacterium abscessus is a rapidly growing agent from the nontuberculous mycobacteria (NTM) complex1-3. It has a broad clinical spectrum, with the cutaneous form being the primary manifestation, characterized by nodules and microabscesses on the skin and in the subcutaneous tissue that form crusted ulcers1,2. Despite its low prevalence, cases are being increasingly reported. These cases occur in outbreaks associated with healthcare settings3. Therefore, its diagnosis can be challenging, requiring an association of clinical reasoning and targeted cultures to identify the specific etiology and, consequently, proposing the appropriate therapeutic approach and right treatment.
The present case report describes the clinical and radiological findings and the microbiological profile of an infection after a cosmetic surgical procedure. For several months, the hypothesis of nonspecific secondary bacterial infection without a defined agent was considered. Considering the infection’s refractoriness to prolonged empirical antimicrobial treatment, imaging and culture tests helped to identify the growth of Mycobacterium abscessus in a deep-tissue sample, allowing specific antibiogram-guided treatment (susceptibility test).
CASE REPORT
A 45-year-old female patient had a history of left-sided radical mastectomy due to breast cancer in 2017, progressing without clinical or surgical complications. Since then, she has been asymptomatic and taking tamoxifen. There was no other relevant medical history. In 2021, the patient underwent breast reconstruction with bilateral implantation of prostheses, also with no complications. The following year she underwent abdominal liposuction and gluteal fat grafting for aesthetic purposes. Five days after this procedure, the patient developed pain and burning sensation in the buttocks, local hyperemia, and desquamation, which led to a diagnosis of a localized skin infection. She underwent prolonged cycles of empirical antibiotic therapy with ciprofloxacin and clindamycin for over 3 months, under the guidance of her assisting surgical team, followed by sulfamethoxazole and trimethoprim for another month, all without success. During this period, she also experienced a weight loss of 12 kg. She was hospitalized after 6 months because of the progressive worsening of the condition.
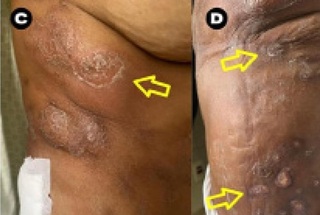
On admission, multiple abscesses with purulent drainage were observed in the buttocks, thighs, abdomen, and breasts, as well as hyperemia of the abdominal wall. Ulcerated and purulent lesions and diffuse necrotic crusts were found on the abdomen, trunk, and posterior region of thighs, and the patient complained of breast pain. There were several ulcers 2-3 cm in diameter, some with areas of necrosis and hyperemic borders, others covered with crusts with desquamation and hyperemia around them. Many ulcers had yellowish purulent secretion, and diffuse hyperchromic nodular lesions were also present in the abdomen and trunk, as well as edema in the abdominal wall and thighs (Figure 1).
Figure 1. Clinical examination of lesions prior to treatment. (A) Multiple ulcer-crusted lesions in the abdomen, with areas of necrosis and necrotic crust (blue arrows), hyperemic borders with a diameter of 2-3 cm, and the presence of abdominal wall edema. (B) Diffuse hyperchromic nodular lesions (red arrows) on the back, trunk, and abdomen. (C) Subcutaneous abscessed lesions with hyperemia and desquamation around them (yellow arrows). (D) Posterior region of the thigh with edema and ulcer-crusted lesions with local purulent discharge (yellow arrows).
Magnetic resonance imaging showed multiple scattered collections throughout the skin tissue from the trunk and pelvis to the thorax, with diffuse enhancement and areas of thickening in the deep fascia, suggesting cellulitis and necrotizing fasciitis. In the soft tissues of the thighs there were also multiple elongated cystic lesions with thin septa in between, extending through the subcutaneous plane to the gluteal regions, closely associated with myofascial planes (Figure 2).
Figure 2. Contrast-enhanced soft-tissue magnetic resonance imaging. (A and B) Coronal sections with multiple collections and abscesses in the abdominal wall and trunk, evidenced by hyposignal on T2-weighted flair images. (C and D) Cross-sections with multiple collections and abscesses on the right dorsum and thigh, evidenced by hyposignal on T2-weighted flair images.
A new broad-spectrum antimicrobial treatment was initiated, this time with glycopeptide antibiotics and carbapenem, to cover both Gram-positive cocci and Gram-negative bacilli from the hospital flora. However, the thoracic lesions progressed, causing incisional dehiscence of the breast implants inserted more than 6 months previously. Periprosthetic breast abscesses were identified by ultrasound, which required surgical drainage and removal of the implants, followed by the placement of a vacuum dressing in the respective pockets.
The empirical broad-spectrum treatment was maintained for another 3 weeks considering negative cultures for aerobic and anaerobic bacteria and for fungi. However, in a specific culture for mycobacteria (in standard Löwenstein-Jensen solid medium and mycobacteria growth indicator tube broth with automated flowmetry), there was growth of Mycobacterium abscessus subspecies abscessus in a single intraoperative sample collected aseptically from what had been considered a closed lesion in a sterile site. Acid-fast bacilli and Mycobacterium tuberculosis polymerase chain reaction (PCR; GenXpert MtbRIF®) were negative in the same sample. The sensitivity profile of M. abscessus is described in Table 1.
| Amikacin | 32 (I) | Doxycycline | 32 (R) |
|---|---|---|---|
| Bedaquiline | 0.06 * | Imipenem | 8 (I) |
| Cefoxitin | 16 (S) | Linezolid | 8 (S) |
| Ciprofloxacin | 16 (R) | Moxifloxacin | 8 (R) |
| Clarithromycin | 32 (R) ** | Sulfamethoxazole / Trimethoprim | 304 / 16 (R) |
| Clofazimine | 0.5 *** | Tigecycline | 2 (S) |
Thus, specific treatment was initiated with amikacin 1 g/day intramuscularly (IM) thrice a week, tigecycline 100 mg/day intravenously (IV), ertapenem 1 g/day (IV), clarithromycin 500 mg orally (PO) every 12 h, and clofazimine 100 mg/day (PO) as a loading dose for 1 month. This was followed by amikacin 1 g/day (IM) thrice a week, clarithromycin 500 mg (PO) every 12 h, and clofazimine 100 mg/day (PO) once a day as a maintenance dose, expected to last 18 months. To date, 7 months of maintenance treatment have been completed, with follow-up at a referral service. Despite the psychological and aesthetic damage, there was a reduction in the number of skin lesions and an improvement in their appearance (the images in Figure 3 were documented after 4 months of treatment), with a reduction in pain and edema. The patient remains afebrile and is under gradual nutritional recovery.
Figure 3. Clinical examination after 4 months of treatment. (A and B) Scarring lesions on the abdomen, back, and thigh (red arrows). (C and D) Abdomen with active lesions, crusted ulcers (yellow arrows), and necrotic ulcers (blue arrow), with hyperemic borders and abdominal wall edema.
DISCUSSION
This report reveals the diagnostic and therapeutic challenges of refractory postoperative infections, reinforcing the possibility of a less common etiology: fast-growing nontuberculous mycobacteria1. In this particular case, the symptoms persisted and worsened over 6 months until the diagnosis was confirmed, which illustrates the importance of directed cultures to allow the identification of the specific etiologic agent and its associated antimicrobial susceptibility profile to determine the appropriate treatment.
The manifestations caused by M. abscessus are characterized by cutaneous and subcutaneous nodules and microabscesses, with a tendency of fistulization of purulent material. They may progress with necrotic or ulcer-crusted forms with a sporotrichoid aspect1,2. These lesions require a differential diagnosis with several other etiologies, including other species of mycobacteria (tuberculous or nontuberculous), malignant syphilis, and even cutaneous lymphomas.
For the diagnosis of NTM infection in closed nodules, aspiration or aseptic biopsy of the lesion is recommended. When there are multiple open lesions, it is advisable to collect several samples1. Because of the possibility that a positive culture may represent sample colonization or contamination, two samples from nonsterile sites or one from a sterile site with a closed cavity are recommended4. The isolation of a rapidly growing mycobacterium from a single sample has its diagnostic value dependent on the clinical context. It is suggestive of active disease when isolated from a sterile sample and when the species is known to be pathogenic. Histopathological examination can also aid in the diagnostic process by characterizing a granulomatous infectious process. Techniques such as immunohistochemistry and/or specific DNA testing (genomic amplification via PCR) can further contribute to the optimization of the results4.
Several outbreaks of mycobacterial infections have been reported after cosmetic surgical procedures and surgical manipulations, including liposuctions, breast augmentations, and larger surgeries like laparoscopic procedures1,3,4. These infections are usually attributed to inadequate cleaning of surgical materials with contaminated water1,3, which is one of the primary risk factors. These mycobacteria are present in soil and water sources, and their hydrophobic lipid cell wall promotes the formation of biofilms in solid materials such as water pipes, catheters, and prosthetic implants3,4.
The initial clinical presentation often leads to a diagnostic hypothesis of a healthcare-associated infection, probably of nonspecific bacterial and nosocomial etiology. In the present case, the infection appeared in the early postoperative period of the liposuction and gluteal fat graft procedure. However, this reasoning can be questioned due to the history of bilateral mammoplasty (and consequent presence of implants). Even if it was performed a few months earlier, the clinical manifestations of infections caused by M. abscessus may appear up to 2 years after the surgical procedure5-8, without a clear definition of the incubation period. Thus, while it is the most likely scenario, it cannot be conclusively inferred that the infection was solely a result of the patient’s most recent procedure.
There are recent reports of emerging fast-growing mycobacterial infections in several countries9-12. In Brazil, there were outbreaks in Bahia, Pará, and Rio Grande do Sul, where M. abscessus, along with M. chelonae and M. fortuitum were identified among the most prevalent agents3. This infection is more prevalent in females due to more frequent aesthetic surgical procedures. Breast implants, liposuction, injection of subcutaneous enzymes, and other procedures such as arthroplasty and abdominoplasty are the procedures with highest-risk3-5. In such cases, it is necessary to consider the possibility of mycobacterial infections to avoid delaying the diagnosis and worsening of the condition, as occurred with this patient. Similar case reports5-8 have also characterized the infection as consisting of nodular lesions and cutaneous and subcutaneous microabscesses, sometimes associated with pulmonary forms with diffuse disease. Other sites of involvement and underlying immunosuppression should also be investigated.
The most effective antimicrobials for the specific treatment of these mycobacteria are macrolides and aminoglycosides4,10,11. Current recommendations for severe cases involving fascia and muscle suggest a 1-3-month loading regimen with amikacin or tigecycline and imipenem or ertapenem, associated with clarithromycin and clofazimine. Maintenance treatment with amikacin and clarithromycin is necessary for another 12-18 months, and these drugs may also be associated with clofazimine and moxifloxacin4. The treatment of NTM infection is prolonged and justified by the fact that NTMs are intracellular agents and have a sensitivity profile that often presents multiple resistances to several classes of antimicrobials3,4,10,11, as was the case of the M. abscessus strain isolated in the present report, among others resistant to macrolides. This hinders therapeutic success, and there is a lack of standardization of the cutoff points for antimicrobial susceptibility as described by the BRCAST (Brazilian Committee on Antimicrobial Susceptibility Testing) and the EUCAST (European Committee on Antimicrobial Susceptibility Testing)4,13.
CONCLUSION
The reported case shows that NTM infection should be considered among the possible diagnoses in cases of refractory postoperative infections. In the context of healthcare-related infections, it is important to alert surveillance agencies and report suspected cases promptly. In terms of diagnosis, it is necessary to collect several samples and culture them in specific media. Regarding treatment, the microbial resistance profile of the isolated agent often presents multiple resistances, even limiting the use of macrolides. Therefore, it is imperative to associate several antibiotics for a long time. Finally, morbidity with aesthetic (and psychological) sequelae certainly cannot be underestimated.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Lyon S, Moura ACL, Grossi MAF, Silva, RCV. Micobacterioses. In: Dermatologia tropical. 1ª ed. Rio de Janeiro: Medbook Editora Científica; 2017. p. 38-41.
2. Nogueira LB, Garcia CN, Costa MSCD, Moraes MB, Kurizky PS, Gomes CM. Non-tuberculous cutaneous mycobacterioses. An Bras Dermatol. 2021;96(5):527-38.
3. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária (Anvisa). Boletim Segurança do Paciente e Qualidade em Serviço de Saúde nº 19 GVIMS/GGTES/Anvisa: Notificação de casos de micobactéria de crescimento rápido (MCR) atualizado - 1998 a 25 de julho de 2023. Brasília (DF): Ministério da Saúde; 2023; [acesso em 2023 Abr 02]. Disponível em: https://app.powerbi.com/view?r=eyJrIjoiYWU2MTQ3OWItZjAwMy00ZTNkLTk2NzAtMzExMjM0O
Dc5M2Y0IiwidCI6ImI2N2FmMjNmLWMzZjMtNGQzNS04MGM3LWI3MDg-1ZjVlZGQ4MSJ9
4. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Recomendações para o diagnóstico e tratamento das doenças causadas por micobactérias não tuberculosas no Brasil. Brasília (DF): Ministério da Saúde; 2021; [acesso em 2023 Abr 02]. Disponível em: https://www.gov.br/aids/pt-br/central-de-conteudo/publicacoes/2021/recomendacoes-para-o-diagnostico-e-tratamento-das-doencas-causadas-por-micobacterias-nao-tuberculosas-no-brasil
5. Sousa PP, Cruz RCS, Schettini APM, Westpha DC. Mycobacterium abscessus skin infection after tattooing - case report. An Bras Dermatol. 2015;90:739-41.
6. Ryu HJ, Kim WJ, Oh CH, Song HJ. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-50.
7. Fitzgerald DA, Smith AG, Lees A, Yee L, Cooper N, Harris SC, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132(5):800-4.
8. Fang RY, Sun QN. Mycobacterium abscessus infections following injection of botulinum toxin. J Cosmet Dermatol. 2020;19(4):817-9.
9. Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18:392-407.
10. Boudehen YM, Kremer L. Mycobacterium abscessus. Trends Microbiol. 2021;29:951-2.
11. Victoria L, Gupta A, Gómez JL, Robledo J. Mycobacterium abscessus complex: a review of recent developments in an emerging pathogen. Front Cell Infect Microbiol. 2021;11:659997.
12. Carvalho NFG, Ferrazoli L, Riveron MBA, Chimara E. Caracterização dos surtos causados pelo grupo Mycobacterium abscessus. Rev Inst Adolfo Lutz. 2012;71(2):228-36.
13. Fröberg G, Maurer FP, Chryssanthou E, Fernström L, Benmansour H, Boarbi S, et al; EUCAST AMST and ESCMYC study groups. Towards clinical breakpoints for non-tuberculous mycobacteria - Determination of epidemiological cut off values for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution. Clin Microbiol Infect. 2023 Jun;29(6):758-64.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















