Gnathostomiasis and cutaneous leishmaniasis: a possible co-infection
DOI:
https://doi.org/10.5935/2764-734X.e202203009Keywords:
Leishmaniasis, Cutaneous, Helminths, Skin UlcerAbstract
Gnathostomiasis is a zoonosis transmitted to humans by eating raw or undercooked foods contaminated with the larvae of Gnathostoma sp. The clinical manifestations of gnathostomiasis vary according to the species, parasite load and affected tissues. Skin lesions are mainly characterized by nodules or areas of edema that affect any topography of the body. Cutaneous leishmaniasis is mainly characterized by a single ulcerated skin lesion caused by different species of Leishmania. In this article we present a case report of Gnasthostoma and Leishmania co-infection, with clinical presentation of an ulcerated skin lesion in the triceps region of the right upper limb that appeared during a trip to Peru. A diagnosis of leishmaniasis was made by detection of amastigotes and at the beginning of treatment, there was an exit of helminth characterized with Gnasthostoma by stereomicroscope. He received treatment with albendazole, and the lesion healed. This is the first report of Gnasthostoma and Leishmania co-infection described in the literature.
Downloads
INTRODUCTION
Gnathostomiasis is a zoonosis caused by the nematode helminth and is transmitted to humans by accidental ingestion of raw or undercooked aquatic animals contaminated with Gnathostoma ssp1 larvae. The disease has been described mainly in Southeast Asia, especially in Thailand and China1. In Latin America, the first record of gnathostomiasis occurred in Mexico in 19702, and in South America, the first report was in Ecuador in 19843, in a series of migratory nodular eosinophilic panniculitis cases. Between 1987 and 1999, four cases of gnathostomiasis in Peru were described as being related to raw fish intake4. The first case report in Brazil occurred in 20095 in a Peruvian patient, but the first autochthonous report occurred in 2012 in a patient from the country’s northern region6. Recently, two other cases from the Brazilian Amazon region have been reported7.
The genus Gnathostoma comprises 13 different species, of which six have been described to date as causing disease in humans: G. spinigerum, G. hispidum, G. binucleatum, G. malaysiae, G doloresi and G. nipponicum8. The life cycle is metaxenic, developing in intermediate hosts (crustaceans and freshwater and saltwater fish) and having domestic and wild mammals as definitive hosts. Paratenic hosts, including, humans, reptiles and birds are also noted1.
Gnathostomiasis clinical manifestations in humans vary depending on species, parasitic load and affected tissues, and there may be visceral or cutaneous manifestations1. Skin lesions are characterized mainly by nodulations or areas of edema that affect any topography of the body, with associated infiltration and erythema, which can be painful and pruriginous. Central nervous system involvement is rare, but this is the most severe form of the disease9.
Depending on the clinical presentation, the cutaneous manifestation of gnathostomiasis may be confused with different skin disorders10. Bacterial infections are the most frequent differential diagnosis; however, cutaneous leishmaniasis with ulcerated presentation may also be confused with gnathostomiasis.
The cutaneous manifestation of leishmaniasis is quite varied. Although, the unique ulcerated lesion is its main clinical form and it also may be confused with other diseases caused by different etiological agents11. Leishmaniasis co-infection with other diseases is not common. Moreover, co-infection of gnathostomiasis with other parasitic diseases is very rare. In this paper, we report a clinical case of co-infection involving gnathostomiasis and cutaneous leishmaniasis.
CLINICAL CASE
A 27-year-old woman, previously healthy, coming from France, sought health services in Sao Paulo due to a skin injury that arose during a trip to Peru, where she consumed ceviche on several occasions. She reported the appearance of an ulcerated skin lesion within 45 days of its evolution in the tricipital region of the right upper limb and was initially treated with enteral antibiotic therapy. When the patient sought medical attention in that country, she was diagnosed with cutaneous leishmaniasis, which was confirmed by the presence of amastigote forms in scraped lesions. Therefore, she was instructed to start treatment as soon as staying long enough in a same city during her trip, which happened to be in Brazil.
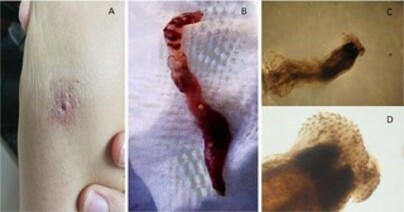
Laboratory tests showed no alterations, and the patient did not have eosinophilia. Initial physical examination showed a shallow, ulcerated cutaneous lesion with a subcutaneous nodule and a serous secretion discharge (Figure 1A). Treatment with intralesional pentavalent antimony was initiated, with local itching and the exit of a larva being reported six days after the first dose. The patient saved the larva (Figure 1B), which was stored in formaldehyde and was sent for taxonomic analysis at the Adolfo Lutz Institute (Figure 1C and 1D). She received treatment with 12 mg/day of ivermectin for two days for empirical treatment of myiasis, and three sequential stool samples were requested, which tested negative for verminosis research. Ultrasound of the skin and soft tissues in the topography of the lesion revealed the presence of a small collection and a part of a dead larva. Treatment of leishmaniasis was completed without complications (three doses of intralesional antimony).
Figure 1. Lesion photograph and photomicrograph of etiological agent. A. A shallow, ulcerated skin lesion, about 1 cm in diameter; B. A 35-mm cylindrical structure removed from the skin lesion, identified as a Gnathostoma; C. Adult worm with cephalic bulb visualization, typical of the genus Gnathostoma, observed in a stereomicroscope with a magnification of 20X; D: View of the cephalic bulb surrounded by thorns, through a stereomicroscope at 40X magnification.
Parasitological examination of the larva identified it as Gnathostoma sp. Treatment of albendazole (800 mg/day) was then performed for 21 days, with no adverse events. There was an important improvement in the ulcerated lesion, which showed complete healing, no nodulation, and no secretion discharge at the end of the treatment. The patient then returned to her home country.
DISCUSSION
Gnathostomiasis is a neglected zoonosis transmitted to humans by ingesting raw aquatic animals infected with a third-stage development larva1. Considered endemic in Latin America12, more than 10,000 cases in Mexico and more than 2000 cases in Ecuador were described between 1986 and 201513. This is the sixth case described in Brazil, not autochthonous to Peru, and is related to the ingestion of raw fish (ceviche).
The presence of amastigote forms of Leishmania in the scraped lesion allowed the diagnosis of cutaneous leishmaniasis (CL), which should not be confused with gnathostomiasis. It is the fact that there was larval exit after the treatment for CL was started, which is apparently the first description of Gnathostoma and Leishmania co-infection in the literature.
One of the main factors related to Gnathostoma infestation is eating habits, mainly the intake of raw fish associated with the presence of intermediate hosts13. The patient traveled through a CL transmission area (Peruvian Amazon and Bolivia), and ingested raw fish. These two epidemiological antecedents, associated with the findings of amastigote forms of Leishmania and the detection of Gnathostoma sp. adult worms, confirm the report of co-infection. Both infections have been reported in the natives of endemic areas and travelers. In this particular case, both gnathostomiasis and leishmaniasis should be considered in the context tropical diseases of the travelers.
The clinical manifestation of gnathostomiasis is classically characterized by nodular migratory panniculitis and eosinophilia due to the migration of the larva through the epidermis, dermis, and subcutaneous tissue. Although, the disease may also manifest as rash, abscess, and isolated nodules. The description of ulcerated lesions is rare10. The cutaneous manifestation of our report was a shallow, ulcerated cutaneous lesion, with a subcutaneous nodule, serous secretion discharge, and an absence of eosinophilia. This was an atypical manifestation, unlike most descriptions in the literature. A possible explanation for this presentation may be the co-infection with Leishmania itself, as it manifests more commonly as a single ulcerated lesion in an exposed area, secondary to a phlebotomine fy bite11. It is known that localized cutaneous leishmaniasis leads to a cell-type immune response pole where there is no eosinophil (TH1) activation15, while diseases caused by helminths induce a TH2 response pole, characterized by humoral immune response and activation of eosinophils16. Therefore the immune response induced by localized CL may have contributed to the absence of eosinophilia that could have been induced by gnathostomiasis.
The diagnosis of CL and gnathostomiasis is mainly based on clinical and epidemiological criteria, while the confirmation of the diseases is made by laboratory, through the detection of amastigote forms and larvae, respectively, at the site of injury1,13. It is noteworthy to highlight the ultrasound discovery of a part of dead larva in a subcutaneous collection in this case, which is not commonly described in the literature1.
Gnathostomiasis is treated with ivermectin or albendazole17. In this case, the patient was first treated with ivermectin due to the initial diagnostic hypothesis of myiasis, but after confirmation of Gnathostoma sp., we switched to albendazole treatment for 21 days, due to the risk of the occurrence of a severe visceral form (CNS gnathostomiasis). The patient had an excellent response to the treatment. CL can be treated with systemic or intralesional medication14. In this case, due to the small ulcerated lesion, treatment with intralesional antimony (3 doses) was choosed and also showed an excellent therapeutic response.
CONCLUSION
We reported the first case of gnathostomiasis and cutaneous leishmaniasis co-infection with atypical presentation of a shallow ulcer and absence of eosinophilia, in which the patient evolved with excellent therapeutic response to both diseases. It is important to highlight that there was a delay in the diagnosis of gnathostomiasis due to ignorance on the part of healthcare professionals, the atypical presentation form and the finding of amastigote forms of Leishmania sp.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access artcle is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009 Jul;22(3):484-92.
2. Peláez D, Pérez-Reyes R. Gnathostomiasis in America. Rev Latinoam Microbiol. 1970 Abr/Jun;12(2):83-91.
3. Ollague W, Ollague J, Guevara de Veliz A, Herrera S. Human gnathostomiasis in Ecuador (nodular migratory eosinophilic panniculitis). First finding of the parasite in South America. Int J Dermatol. 1984 Dez;23(10):647-51.
4. Villar de Cipriani E. Paniculitis migratoria eosinofílica en el Perú: Gnathostoma como agente causal. Rev Peru Med Exp Salud Publica. 2003 Out/Dez;20(4):220-2.
5. Dani CMC, Mota KF, Sanchotene PV, Maceira JP, Maia CPA. Gnatostomíase no Brasil: relato de caso. An Bras Dermatol [Internet]. 2009 Ago; [citado 2021 Feb 8]; 84(4):400-4. Disponível em: https://www.scielo.br/j/abd/a/fc7GmYgcBXnDbx3vZ58bLyN/?lang=pt
6. Vargas TJS, Kahler S, Dib C, Cavaliere MB, Jeunon-Sousa MA. Autochthonous gnathostomiasis, Brazil. Emerg Infect Dis. 2012 Dez;18(12):2087-9.
7. Haddad Junior V, Oliveira ÍF, Bicudo NP, Marques MEA. Gnathostomiasis acquired after consumption of raw fresh water fish in the Amazon region: are port of two cases in Brazil. Rev Soc Bras Med Trop. 2021;54:e20200127.
8. Diaz JH. Gnathostomiasis: an emerging infection of raw fish consumers in Gnathostoma nematode-endemic and nonendemic countries. J Travel Med. 2015 Set/Out;22(5):318-24.
9. Liu G H, Sun MM, Elsheikha HM, Fu YT, Sugiyama H, Ando K, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020 Dez;13(1):616.
10. Bravo F, Gontijo B. Gnathostomiasis: an emerging infectious disease relevant to all dermatologists. Ann Bras Dermatol. 2018 Mar;93(2):172-80.
11. Goto H, Lindoso JAL. Cutaneous and mucocutaneous leishmaniasis. Infect Dis Clin North Am. 2012 Jun;26(2):293-307. DOI: https://doi.org/10.1016/j.idc.2012.03.001
12. Leroy J, Cornu M, Deleplancque AS, Loridant S, Dutoit E, Sendid B. Sushi, ceviche and gnathostomiasis - a case report and review of imported infections. Travel Med Infect Dis. 2017 Nov/Dez;20:26-30.
13. Nawa Y, Mallewong W, Intapan PM, Camacho SPD. Gnathostoma. In: Xiao L, Ryan U, Feng Y, eds. Biology of foodborne parasites. Boca Raton: CRC Press; 2015. p. 420-41.
14. Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. IntJ Infect Dis. 2010 Dez;14(12):e1032-9.
15. Scott P, Novais FO. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol. 2016 Jul;16(9):581-92.
16. Moreau E, Chauvin A. Immunity against helminths: interactions with the host and the intercurrent infections. J Biomed Biotechnol. 2010;2010:428593.
17. Kraivichian K, Nuchprayoon S, Sitichalernchai P, Chaicumpa W, Yentakam S. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004 Nov;71(5):623-8.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















