Severe malaria with encephalopathy and acute kidney failure
DOI:
https://doi.org/10.5935/2764-734X.e202204013Keywords:
Malaria, Acute kidney injury, Acute febrile encephalopathy, Time-to-treatment, Case ReportAbstract
The Legal Amazon region concentrates 99% of malaria cases in Brazil, where Plasmodium vivax is the main etiological agent (90% of cases). This report aims to demonstrate the main role of the patient’s epidemiological history in an acute febrile jaundice syndrome - it’s a case of malaria diagnosed in a patient coming from Benin, Africa, where all cases correspond to Plasmodium falciparum infection. The diagnosis and introduction of appropriate treatment with artesunate were delayed, in such a way that the patient progressed to severe forms of the disease (acute kidney injury and encephalopathy), followed by death.
Downloads
INTRODUCTION
The Brazilian Legal Amazon region (Acre, Amapá, Amazonas, Maranhão, Mato Grosso, Pará, Rondônia, Roraima, and Tocantins) concentrates 99% of malaria cases in the country, with Plasmodium vivax being the main etiologic agent (90% of cases). Of the remaining 1% of cases reported in extra-Amazonian regions, 1/3 are of autochthonous origin (Atlantic Forest region) and 2/3 are imported from endemic Brazilian states or from other countries2,3. Plasmodium falciparum is the main etiologic agent in cases imported from Africa and Asia, respectively accounting for 90% and 60% of the cases in those continents1,2. Due to the low incidence of extra-Amazonian malaria, most health services outside these regions have no resources to diagnose and treat this disease, and suspected cases are transferred to tertiary referral centers2,3.
The objective of this report is to highlight the importance of epidemiological background in the care of patients with acute febrile jaundice syndrome (in this case, coming from a place where malaria is endemic) to promptly establish the diagnosis and early treatment, preventing disease progression to severe forms with greater lethality.
CASE REPORT
This is the case of a 20-year-old man born in Dhaka, Bangladesh, who had lived for three months in Porto Novo, Benin, before arriving five days ago in São Paulo, Brazil. His epidemiological background is from a country where the Annual Parasite Index (API) is greater than 300 and Plasmodium falciparum is the agent responsible for all cases of malaria1.
At disease onset, two days before his arrival in Brazil, he presented mild and nonspecific symptoms such as unmeasured fever, myalgia, abdominal pain, and jaundice. Four days after onset, he sought medical care at a Basic Health Unit already in Brazil, being discharged with symptomatic medication. The following day, he sought emergency care, being also discharged with symptomatic medication after medical evaluation. On the sixth day of symptom onset, he progressively worsened to the severe form of the disease, being admitted to the emergency room of a Municipal Hospital. In that service, the patient presented jaundice 3+/4+, fever (38º C), tachycardia (111 bpm), hypotension (BP 60/30 mmHg) and torpor. Laboratory tests on admission showed thrombocytopenia (Table 1) and the presence of trophozoites in red blood cells (RBC) on blood smear, establishing the diagnosis of severe malaria. Due to the local lack of specific medication for the treatment (artesunate), the patient was transferred to a referral hospital, being admitted to the Emílio Ribas Institute of Infectious Diseases on day seven after the onset of symptoms. The patient was in poor general condition, confused, and drowsy (Glasgow coma scale 12), pale 3+/4+, jaundiced 3+/4+, and febrile (37.8° C). Laboratory results are listed in Table 1. Treatment for severe malaria was instituted with 2.4 mg/kg/dose intravenous artesunate twice daily (every 12 hours) associated with 600 mg intravenous clindamycin every eight hours. The patient rapidly progressed with neurological worsening (decreased level of consciousness — Glasgow Scale 8) and arterial hypotension, requiring orotracheal intubation. Sedation was instituted with fentanyl and propofol, and norepinephrine was started to normalize blood pressure levels.
| Test | Reference range | Admission test at the Municipal Hospital (6 days after symptom onset) | Admission test at the IIER ICU (7 days) | Second day at the IIER (8 days) |
|---|---|---|---|---|
| Hemoglobin | 13–18 g/dl | 10.5 | 6.1 | 6.9 |
| Platelets | 140–450 mil/mm3 | 18.000 | 25.000 | 23.000 |
| Creatinine | 0.72–1.25 mg/dl | 1.00 | 0.87 | 1.88 |
| Urea | 15–55 mg/dl | 89 | 90 | 115 |
| HCO3 | 22–26 mmol/L | - | 16.9 | 14.9 |
| PH | 7.35–7.45 | - | 7.31 | 7.23 |
| Sodium | 135–147 mmol/l | 125 | 129 | 132 |
| Lactate | 4.5–14.4 mg/dl | - | 59 | 55 |
| Total bilirubin | 0.2–1.2 mg/dl | - | 19.38 | 23.37 |
| direct | <0.5 mg/dl | - | 12.5 | 15.3 |
| indirect | 0.2–0.7 mg/dl | - | 6.88 | 7.97 |
| Lactate dehydrogenase (DHL) | 125–220 U/L | - | 846 | 1747 |
| C-reactive protein | <5.00 mg/L | 26.3 | 151.8 | 208.8 |
| Blood glucose | 70–99 mg/dl | - | 83 | 63 |
On the second day of hospitalization, the patient’s laboratory results worsened (Table 1) and he presented oliguria (50 ml of urine in 12 hours), undergoing renal replacement therapy (RRT). He progressed with refractory shock even with increased doses of vasoactive drugs, followed by cardiorespiratory arrest in asystole, with no response to the usual resuscitation maneuvers.
DISCUSSION
The API estimates the risk of annual occurrence of malaria. This risk is considered low if API is less than 10, medium with values between 10–49, and high when greater than 504. In Benin, where the patient lived for three months before arriving in Brazil, the API is greater than 300 (Figure 1), with Plasmodium falciparum being the only agent responsible for all cases1.
Figure 1. Annual Parasite Index (API) and Predominant Species in Benin (2017).
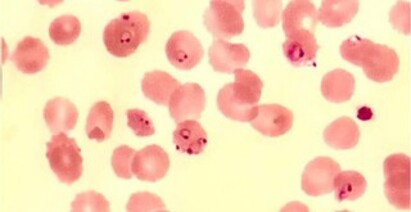
In countries with medium and high API, malaria should be considered the main diagnostic hypothesis in a patient with febrile jaundice syndrome. The diagnosis must be established through direct visualization of the parasite in peripheral blood smears (Figures 2 and 3) 2,3, but this happened in our case only seven days after the onset of symptoms, with treatment starting on the eighth day. Due to the delayed treatment, the patient progressed to severe forms of malaria with acute kidney injury (AKI), arterial hypotension and encephalopaty.
Figure 2. Blood smear collected on ICU admission showing trophozoites in RBC.
Figure 3. Thick blood smear on ICU admission showing countless trophozoites.
AKI is present in about 40% of severe malaria cases due to P. falciparum in endemic regions5. The pathophysiology of renal involvement includes cytoadherence of infected erythrocytes obstructing the kidney microcirculation. In addition, hypovolemia increases the sympathetic tone, activates the renin-angiotensin-aldosterone system and increases vasopressin levels, leading to important renal vasoconstriction. These two mechanisms cause and perpetuate acute tubular necrosis, the main histopathological finding of AKI in malaria. In addition, the process of immune complex and erythrocyte remains deposition in the mesangial membrane of the glomerulus causes acute glomerulonephritis6,7. Clinical presentations of AKI in malaria include oliguria (46–76%) and choluria associated with laboratory findings of severe metabolic acidosis, hyperbilirubinemia, thrombocytopenia, and hemolysis. Patients progressing with the need for RRT present a mortality rate of approximately 75%8,9.
Our patient also presented another severe malaria condition — encephalopathy. Considered the main complication of the disease, it can cause neurological sequelae with a mortality rate of 15 and 20%. The clinical presentation of malarial encephalopathy includes headache, irritability, agitation, hallucinations, seizures, confusion, and coma. Focal signs and cranial nerve involvement may be occasionally present9. In this report, encephalopathy due to severe malaria was observed on ICU admission at the referral hospital as the patient was already confused and drowsy, worsening 24 hours with lowered level of consciousness requiring orotracheal intubation. Infected erythrocyte sequestration is one of the main mechanisms involved in the pathogenesis of malaria encephalopathy9. Uninfected red blood cells (RBC) can agglutinate from binding with infected erythrocytes (autoagglutination), forming rosettes. Platelet mediation and polymorphic RBC surface antigen (PfEMP1) binding with CD36 and ICAM-1 receptors expressed on the surface of host endothelial cells can also occur10. The process of erythrocyte agglutination and sequestration culminates in reduced microvasculature blood flow, thus leading to cerebral hypoxia. However, ischemic injuries are not common.
Another determinant mechanism in the onset of encephalopathy is the presence of exacerbated immune response mediated by TH1 lymphocytes, responsible for the production of pro-inflammatory cytokines such as TNF-alpha and interferon-gamma. These cytokines, in turn, stimulate the synthesis of nitric oxide in brain tissue, causing neuronal dysfunction due to their excitotoxicity10.
Metabolic changes such as hypoglycemia and anemia can further worsen the neurological condition9,11. In the present case, the patient presented hypoglycemia (63 mg/dl) and progressive hemolytic anemia (hemoglobin 6,9; LDH, 1,747) on admission tests, which are aggravating factors for cerebral hypoxia. Finally, infected erythrocyte sequestration increases blood volume in the central nervous system, causing cerebral edema that can be visualized on skull tomography or magnetic resonance imaging. However, intracranial hypertension is not commonly seen in adult patients9, 10, 11.
Artesunate is the treatment of choice for severe malaria. This drug is in the artemisinin class that acts in the erythrocytic phase of malaria, with gametocidal and schizonticidal action through the inhibition of the PFATP enzyme, thus reducing calcium efflux for organelles and promoting parasitic death2,3. Artesunate should be prescribed intravenously at a dose of 2.4 mg/kg (every 12 hours) in three initial administrations. Maintenance treatment consists of the same drug and the same dose administered every 24 hours for a maximum of seven days, in order to transitioning to oral treatment with artemether 40 mg + lumefantrine 240 mg every 12 hours for three days, associated with primaquine 45 mg on the first day2,3. It is worth mentioning that the time elapsed between disease onset and treatment is directly related to prognosis and progression11.
Clindamycin can also be used intravenously in the treatment of malaria. It acts in the erythrocytic phase of the parasitic cycle, with schizonticidal activity inhibiting protein synthesis. However, it is only indicated when artesunate is not available. The recommended dose is 20 mg/kg/day intravenously every eight hours for seven days2. In the present report, clindamycin was individually associated due to case severity and for being intravenously available.
CONCLUSION
This case report aims at demonstrating the importance of the patient’s epidemiological background and its correlation with the clinical history in cases of acute febrile jaundice syndrome, especially for promptly diagnosing imported cases of malaria. The administration of specific treatment in the initial phase of the disease is essential to avoid progression to severe forms and to decrease mortality rates.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access artcle is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work and authorship are properly cited.”
References
1. World Health Organitazion (WHO). World malaria report 2019 [Internet]. Geneva: WHO; 2019. [acesso em 2021 Set 05]. Disponível em: https://www.who.int/publications/i/item/9789241565721
2. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Guia prático de tratamento da malária no Brasil. Brasília (DF): Ministério da Saúde; 2020. [acesso em 2021 Set 05]. Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/guia_tratamento_malaria_brasil.pdf
3. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Manual de vigilância, prevenção e controle de zoonoses. Brasília (DF): Ministério da Saúde; 2016. [acesso em 2021 Set 05]. Disponível em: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/publicacoes-svs/zoonose/manual-zoonoses-normas-2v-7julho16-site.pdf/view
4. Ministério da Saúde (BR). DATASUS - Indicadores de dados básicos Brasil: índice parasitário anual (IPA) de malária [Internet]. Brasília (DF): Ministério da Saúde; 2021; [acesso em 2020 Set 07]. Disponível em: http://tabnet.datasus.gov.br/cgi/idb2000/fqd04.htm#:~:text=Esse%20risco%20est%C3%A1%20relacionado%20%C3%A0,(%E2%89%A550%2C0)
5. Koopmans L, Wolfswinkel M, Hesselink, D, Hoorn E, Koelewijn R, Van Hellemond JJ, et al. Acute kidney injury in imported Plasmodium falciparum malaria. Malaria J. 2015;14:523. doi: 10.1186/s12936-015-1057-9.
6. Nguansangiam S, Day NPJ, Hien TT, Mai NTH, Chaisri U, Riganti M, et al. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health. 2007 Set;12(9):1037-50.
7. Mishra SK, Das BS. Malaria and acute kidney injury. Semin Nephrol. 2008 Jul;28(4):395-408.
8. Plewes K, Royakkers AA, Hanson J, Hasan MMU, Alam S, Ghose A, et al. Correlation of biomarkers for parasite burden and immune activation with acute kidney injury in severe falciparum malaria. Malaria J. 2014 Mar;13:91. doi: 10.1186/1475-2875-13-91.
9. Mishra SK, Newton CRJC. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009 Abr;5(4):189-98.
10. Queiroz NL, Teixeira MM, Teixeira AL. Imunopatogênese da malaria cerebral. Rev Bras Neurol. 2008;44(1):13-9.
11. World Health Organitazion (WHO). Severe malaria. Trop Med Int Health. 2014 Set;19(Supl 1):S7-S131. doi: 10.1111/tmi.12313_2.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















