Challenges in the diagnosis and management of neuroparacoccidioidomycosis associated with pulmonary tuberculosis
DOI:
https://doi.org/10.5935/2764-734X.e20240135Keywords:
Invasive Fungal Infections, Central Nervous System Fungal Infections, Paracoccidioidomycosis, Case ReportAbstract
Paracoccidioidomycosis is one of the most relevant systemic mycoses in Latin America, especially in Brazil where it has the highest prevalence. Central nervous system involvement is a serious complication of the disease and requires early identification and treatment to achieve an effective clinical cure. Current treatments are prolonged, with considerable toxicity and, adding to the severity of the cases, result in high morbidity rates. The present report refers to a patient with no known previous immunodepression who presented with central nervous system infection by the fungus Paracoccidioides spp,. associated with pulmonary only tuberculosis. Although initially misdiagnosed, the disease was confirmed by pathology and the initial treatment consisted of a loading dose of liposomal amphotericin B, followed by consolidation with high-dose fluconazole. The patient had a good clinical course.
Downloads
INTRODUCTION
Among the infectious diseases affecting the central nervous system (CNS), paracoccidioidomycosis (PCM) is of high prevalence in Brazil. It is a systemic mycosis caused by a thermally dimorphic fungus, related to agricultural activities, whose incidence and prevalence are underestimated due to its mandatory reporting being restricted to some states (only since 2021) and not throughout the national territory. It is estimated that around 80% of PCM cases worldwide are recorded in Brazil, particularly in the states of São Paulo, Paraná, Rio Grande do Sul, Goiás, and Rondônia1. CNS involvement by Paracoccidioides spp. causes a potentially serious granulomatous disease that is more frequent in male patients aged over 30 years, mildly immunocompromised individuals, and those with chronic pneumopathy2.
Here we report the case of an immunocompetent patient with neuroparacoccidioidomycosis (NPCM) and concomitant pulmonary tuberculosis, whose clinical condition lasted for months until the correct diagnosis was made and appropriate pharmacological treatment was initiated.
CASE REPORT
The patient was a 46-year-old white single man born in State of São Paulo (SP) inland, previously dyslipidemic, former smoker (22 packs/year), and alcoholic (two to three shots of cachaça a day). He worked as a truck driver in the Campinas-SP macroregion and never left the state. He denied habits, such as eating bushmeat, but mentioned frequent contact with soil throughout his childhood, having eaten armadillo meat and, as an adult, having worked in a pottery factory as a secondary profession for a short period of time.
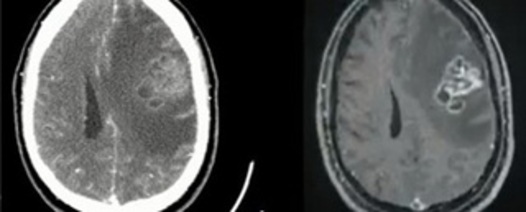
His initial clinical complaint was progressive right hemiparesis, with no implications of his basic and instrumental activities of daily living, which was the reason he had not sought medical attention since the onset of the condition. However, over the course of approximately 40 days, he developed right-sided deviation of the labial commissure and subsequently generalized tonic-clonic seizure, with loss of sphincter control, and was then taken to the emergency department as a matter of urgency. On this occasion, he underwent computed tomography (CT) of the skull which showed a tumor in the left frontal region, with adjacent edema that was better seen on magnetic resonance imaging (MRI) (Figures 1A and 1B).
Figure 1. A: CT scan of the skull showing a tumor in the left frontal region. Figure 1B: MRI showing the expansive lesion with large adjacent edema.
The first hypothesis was metastatic CNS cancer and chest CT was indicated to search for the primary site, which showed a mass in the apico-posterior segment of the upper lobe of the left lung (Figure 2), extending to the corresponding suprahilar region.
Figure 2. Chest CT scan showing a heterogeneous opacity in the left upper lobe extending to the hilum, with partially well-defined contours and boundaries, and oval morphology measuring 3.2 x 4.5 x 2.6 cm3.
The patient underwent a stereotactic biopsy of the brain, whose anatomopathological analysis showed “an inflammatory process characterized by a lymphohistiocytic infiltrate forming granulomas, with dense accumulations of neutrophils and pyocytes, areas of necrosis and numerous round fungal structures of different sizes, consistent with Coccidioides immitis.” Azole antifungal therapy with itraconazole 200 mg/day was started and the patient was discharged shortly afterward - the associated use of corticoids was not reported. He progressed satisfactorily for the following four months, until he had a recurrence of neurological symptoms, namely headache and mild cognitive deficit, with loss of recent memory. He was therefore referred for assessment and follow-up in a specialized clinic where he was readmitted for further investigation.
This time he was admitted in good general condition, hemodynamically stable and eupneic on room air, alert, and with preserved cognition. However, on neurological examination there was a slight alteration in the gait on the right side, with motor strength of grade 4 on right and grade 5 on left, with no significant changes in deep reflexes. Considering the previously established diagnosis of coccidioidomycosis, it was decided to change the treatment to intravenous fluconazole (900 mg/day) as it has better penetration in the CNS, and to update the head and chest images. Compared to the CT findings of the first hospitalization, 4 months previously, there was partial regression of the edema of the head lesion, without significant change in the lung image. Analysis of the cerebrospinal fluid (CSF) obtained by lumbar puncture showed normal biochemical and cellular parameters. The CSF test for acid-fast bacilli (AFB) was negative, as was the rapid molecular test for Mycobacterium tuberculosis (RMT-TB). Serologies for systemic mycoses (paracoccidiodomycosis, aspergillosis, and histoplasmosis) were collected and the results were negative, as were the serologies for HIV, syphilis, and viral hepatitis.
In addition, with the aim of excluding the possibility of an underlying lung tumor (considering that the presence of a mass is not a common presentation in coccidioidomycosis), bronchoscopy was performed with bronchoalveolar lavage (BAL) and transbronchial biopsy. The endoscopic view was normal and AFB testing in BAL was negative, but RMT-TB performed with the GeneXpertMtbc/RIF® system was positive, with low levels of detection, sensitive to rifampicin. The final mycobacteria culture result showed the growth of M. tuberculosis and the sensitivity test confirmed rifampicin and isoniazid sensitivity. BAL cultures for bacteria and fungi were negative, while transbronchial biopsy showed only nonspecific chronic inflammation in the bronchial mucosa and focal anthracosis in the adjacent lung parenchyma, without granulomas. Treatment for pulmonary tuberculosis was initiated with the basic regimen recommended by the Brazilian Ministry of Health, considering the patient’s weight: four co-formulated RHZE tablets (each tablet containing rifampicin 150 mg, isoniazid 75 mg, pyrazinamide 400 mg, and ethambutol 275 mg).
The patient remained clinically stable and showed improvement of right hemiparesis, which was attributed to the use of fluconazole. He was discharged after 30 days of hospitalization for early outpatient follow-up with oral antifungal therapy (fluconazole 900 mg/day) and RHZE.
On his return to the outpatient clinic, the patient brought the paraffin block of the stereotactic biopsy that had been performed during the first hospitalization, at the request of the current medical team, for histological review. The new report described the findings as follows: “Extensive areas of necrosis permeated by a mixed inflammatory infiltrate composed of lymphocytes and histiocytes, with some granulomatous reaction, and polymorphonuclear cells. Amidst of the infiltrate, numerous yeast-like fungal structures are observed that vary greatly in size, some exhibiting multiple sporulation, visualized through histochemical staining by the Grocott’s method. Negative test for AFB when stained using the Ziehl-Neelsen method. Conclusion: Paracoccidioidomycosis, with positive immunohistochemistry for Paracoccidioides brasiliensis (Figure 3).”
Figure 3. Histological section using Grocott’s stain showing numerous yeast-like fungal structures with great variability in size, some exhibiting multiple sporulation or “ship’s wheel” sprouting (arrows).
Given this new diagnosis, the patient was readmitted for induction treatment with liposomal amphotericin B: he received a total dose of 40 mg/kg over seven days and was discharged on oral fluconazole 900 mg/day. He remained without recurrence of motor neurological symptoms after six months of outpatient follow-up, with imaging control showing slow but progressive improvement of the brain lesion (Figure 4). The treatment for tuberculosis was completed within six months, and the patient remained asymptomatic from a respiratory point of view. The proposed schedule is to maintain outpatient follow-up for at least another two years, with quarterly clinical assessments and radiological examinations repeated every six months.
Figure 4. Control imaging (MRI of the skull) after three months of treatment, showing partial radiological improvement compared to the images obtained at the beginning of the condition.
DISCUSSION
In the last two decades there has been a significant increase in fungal infections affecting the CNS in immunosuppressed patients3, but their real incidence in immunocompetent patients is unknown; clinical studies do not always include complete neurological assessments with laboratory and imaging tests, and autopsies, which obviously only present evidence of disseminated disease that culminated in death, do not usually include the spinal cord3.
In any case, the diagnosis and treatment of these conditions are quite challenging. The clinical manifestations are often non-specific and difficult to distinguish from other infectious and non-infectious conditions, especially cerebrovascular and neoplastic diseases4. An accurate diagnosis and appropriate therapeutic measures are decisive for the clinical course of patients and can prevent unfavorable outcomes, such as death or sequelae that have a significant impact on the quality of life of those affected5. Once the diagnostic challenges have been overcome, the next step is to choose the appropriate medication for each infection, as there needs to be good penetration of the blood-brain barrier and effective antifungal activity against the specific agent6.
CNS involvement in patients with paracoccidioidomycosis occurs in 9.9% to 27.3% of cases2. This variation is explained by the different diagnostic methods used to identify the neurological lesions caused by the fungus and indicates that the incidence of NPCM is probably higher and underestimated, because that diagnostic and staging resources are often limited. Despite being commonly associated with immunocompromised hosts, systemic mycoses with dissemination to the CNS also affect apparently immunocompetent individuals, as in the present case. In any case, these presentations lead to significant morbidity and mortality, often due to delayed diagnosis and consequent delay in starting the appropriate treatment4.
Inhalation of fungal propagules is a common form of PCM contamination, and the initial spread from the alveoli occurs via hematogenous and lymphatic routes, i.e., the disease in the CNS is secondary. NPCM can occur in isolation but concomitant involvement of other organs is common: the lung is usually affected in 61% to 83% of these cases 7. In addition, paracoccidioidomycosis is often associated with other chronic lung diseases, such as tuberculosis. The frequency of this association ranges from 5.5% to 19% and can occur simultaneously or sequentially8. This also leads to diagnostic delays due to the similar clinical presentation and the greater tendency for tuberculosis to be privileged in clinical reasoning, often even deserving empirical treatment without diagnostic confirmation. It is also common for investigations to be closed as soon as a possible etiological agent has been identified, without there being any suspicion of coinfection. This phenomenon should always be considered when the clinical course is characterized by an apparent initial improvement (due to the treatment of one of the infections) followed by unexpected stagnation or worsening of the condition.
In addition to tuberculosis, different invasive fungal diseases, most notably endemic mycoses, can lead to a variety of similar clinical manifestations, and in this context it is strategic to look for other epidemiological risk factors 9. In the case reported herein, for example, the patient did not have well-defined risk factors for coccidioidomycosis (his first diagnosis) such as visiting endemic regions. The patient reported eating armadillo meat as a child, but the geographical region and the time elapsed until the onset of symptoms are not consistent with infection by Coccidioides spp., whose incubation period is short, normally between one and four weeks; incubation periods of years are only seen in immunocompromised patients10, which was not the case.
PCM etiology was confirmed through the histological review of the brain lesion biopsy, with specific staining to detect the pathogen; however, a misdiagnosis is not uncommon when the investigation is solely based on the morphological structure of microorganisms, as was the case6. PCM presents yeast-like structures with multiple sporulation that typically have a “ship’s wheel” appearance; similarly, coccidioidomycosis presents spherules containing multiple endospores that, in their most immature forms, can be confused with the two species currently recognized as causing PCM (P. brasiliensis and P. lutzii)3. In addition, Coccidioides species are morphologically indistinguishable, with molecular methods being required to identify them11. While C. immitis is the most prevalent species in North America, C. posadasii is of greater importance in the rest of the continent, with endemic areas in northeastern Brazil, especially in the states of Ceará, Piauí, Maranhão, and Bahia9.
NPCM presents granuloma as the most frequent imaging pattern, which helps in differentiating it from other causes of single or multiple brain lesions, although it is not very specific12. On CT images, this lesion is revealed as hypodense, with irregular margins, irregular peripheral enhancement of variable thickness, and associated perilesional edema. On MRI, a strong hypointense signal is usually observed on T2-weighted images due to increased iron deposition as a result of fungal elements and hemorrhage13. More recently, Brazilian researchers have described the “star of Bethlehem sign” as an eccentric mural nodule with post-contrast enhancement observed in lesions larger than 1.2 cm that disappears with treatment14.
Multiple expansive lesions in the brain parenchyma (considered the pseudotumoral form) are more frequent in NPCM, reaching 87.5% of cases in some studies. That is why the most common symptoms are seizures and focal neurological signs, with or without headache 7,15, as in the present case. On the other hand, CNS infection by coccidioidomycosis manifests more often as meningitis, with leptomeningeal enhancement of the basal cisterns being the most common imaging finding (91%), followed by hydrocephalus (78%) and spinal abnormalities (86%). Thus, the clinical picture of coccidioidomycosis is characterized mainly by progressive holocranial headache, cognitive and gait disorders and, in the absence of adequate therapeutic intervention, progression to decreased level of consciousness11,16.
Culture is another important method for confirming the diagnosis of fungal infections in the CNS. However, samples can be inadvertently contaminated with bacteria that decrease the sensitivity of the test, which is why all culture media should contain antibiotics 9. Paracoccidioides spp. is a slow-growing fungus and cultures can take three to four weeks to become positive. It should be emphasized that prior suspicion of PCM is crucial and that the local laboratory should be notified about the cultivation time of the samples and take the appropriate biosafety measures regarding the potential biological hazard of handling this fungus9.
The treatment of NPCM should take into account factors, such as the availability of the medications in the clinic, the prescribers’ experience with the drugs that act against this fungus, the patient’s clinical condition and comorbidities, as well as the degree of involvement of the disease1,17. In addition, attention must be paid to the particularities of each medication, especially its bioavailability and penetration of the blood-brain barrier. In this context, sulfonamides, particularly the sulfamethoxazole-trimethoprim combination, have been used the most1. Another regimen used in our clinic is fluconazole in high doses (600 to 1200 mg/day), as it also has excellent penetration of the blood-brain barrier and is widely available in the Brazilian Unified Health System. Conventional amphotericin and its lipid formulations are reserved for the most severe conditions18.
CONCLUSION
This case highlights the challenges in diagnosing Paracoccidioides spp CNS infection in an immunocompetent individuals. The symptoms are unspecific and similar to those seen in other diseases, whether infectious or not. Images of expansive lesions in the CNS concomitant with radiographic findings of pulmonary involvement may be suggestive of metastatic cancer, which is why it is imperative to perform a biopsy of the lesions. However, our patient’s initial diagnosis was incorrect because it was exclusively histological and the condition was attributed to another pathogenic fungus. The concomitant presence of pulmonary TB, another relatively frequent finding in clinical practice, also contributed to confusion in the diagnostic process. These hindrances lead to delays in providing appropriate treatment to patients. Thus, we reinforce the importance of a more comprehensive investigation that includes the analysis of epidemiological risk factors and clinical history and performing complementary and specific tests at an early stage.
“This case report deserved an official declaration of acknowledgement and ethical approval by its institution of origin and was peer-reviewed before publication, whilst the authors declare no fundings nor any conflicts of interest concerning this paper. It is noteworthy that case reports provide a valuable learning resource for the scientific community but should not be used in isolation to guide diagnostic or treatment choices in practical care or health policies. This Open Access article is distributed under the terms of the Creative Commons Attribution License (CC-BY), which allows immediate and free access to the work and permits users to read, download, copy, distribute, print, search, link and crawl it for indexing, or use it for any other lawful purpose without asking prior permission from the publisher or the author, provided the original work and authorship are properly cited.”
References
1. Shikanai-Yasuda MA, Mendes RP, Colombo AL, Telles FQ, Kono A, Paniago AMM, et al. II Consenso Brasileiro em Paracoccidioidomicose - 2017. Epidemiol Serv Saúde. 2018;27(spe):e0500001. DOI: 10.5123/S1679-49742018000500001
2. Fagundes-Pereyra WJ, Carvalho GTC, Góes ADM, Silva FCL, Sousa AA. Paracoccidioidomicose do sistema nervoso central: análise de 13 casos. Arq Neuro-Psiquiatr. 2006;64(2a):269-76. DOI: 10.1590/S0004-282X2006000200018
3. Hahn RC, Hagen F, Mendes RP, Burger E, Nery AF, Siqueira NP, et al. Paracoccidioidomycosis: current status and future trends. Clin Microbiol Rev. 2022 Sep;35(4):e00233-21. DOI: 10.1128/cmr.00233-21
4. Cambruzzi E, Pêgas KL, Nascimento GBC, Silva JNAM, Zandoná NB, Kus WP, et al. Multifocal pseudotumorous form of neuroparacoccidioidomycosis in an immunocompetent patient: a clinicopathological review based on a case report. Arq Bras Neurocir 2021;40(2):e195-e9. DOI: 10.1055/s-0040-1719005
5. Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol. 2018 Apr;17(4):362-72. DOI: 10.1016/S1474-4422(18)30030-9
6. Nathan CL, Emmert BE, Nelson E, Berger JR. CNS fungal infections: a review. J Neurol Sci. 2021 Mar;422:117325. DOI: 10.1016/j.jns.2021.117325
7. Vilela MDC, Pedroso VSP, Teixeira AL. Paracoccidioidomicose com comprometimento do sistema nervoso central: revisão sistemática da literatura. Rev Soc Bras Med Trop. 2009 Dec;42(6):691-7. DOI: 10.1590/S0037-86822009000600016
8. Quagliato Junior R, Grangeia TA, Massucio RA, Capitani EM, Rezende SM, Balthazar AB. Association between paracoccidioidomycosis and tuberculosis: reality and misdiagnosis. J Bras Pneumol. 2007 Jun;33(3):295-300. DOI: 10.1590/s1806-37132007000300011
9. Peçanha-Pietrobom PM, Tirado-Sánchez A, Gonçalves SS, Bonifaz A, Colombo AL. Diagnosis and treatment of pulmonary coccidioidomycosis and paracoccidioidomycosis. J Fungi (Basel). 2023;9(2):218. DOI: 10.3390/jof9020218
10. Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016 Jul;63(6):e112-e46. DOI: 10.1093/cid/ciw360
11. Deus Filho A. Capítulo 2: coccidioidomicose. J Bras Pneumol. 2009 Sep;35(9):920-30. DOI: 10.1590/S1806-37132009000900014
12. Oliveira VF, Magri MMC, Levin AS, Silva GD. Systematic review of neuroparacoccidioidomycosis: the contribution of neuroimaging. Mycoses. 2023 Feb;66(2):168-75. DOI: 10.1111/myc.13525
13. Costa RS, Cruz Junior LCH, Souza SR, Ventura N, Corrêa DG. Insights into magnetic resonance imaging findings in central nervous system paracoccidioidomycosis: a comprehensive review. Res Rep Trop Med. 2023;14:87-98. DOI: 10.2147/RRTM.S391633
14. Santana LM, Peçanha PM, Falqueto A, Kruschewsky WLM, Grão-Velloso TR, Gonçalves SS, et al. “Star of Bethlehem sign” in the analysis of the evolution of brain lesions during and after treatment for neuroparacoccidioidomycosis. Radiol Bras. 2023 Jul/Aug;56(4):195-201. DOI: 10.1590/0100-3984.2023.0030
15. Rosa Junior M, Amorim AC, Baldon IV, Martins LA, Pereira RM, Campos RP, et al. Paracoccidioidomycosis of the central nervous system: CT and MR imaging findings. Am J Neuroradiol. 2019;40(10):1681-8. DOI: 10.3174/ajnr.A6203
16. Jackson NR, Blair JE, Ampel NM. Central nervous system infections due to coccidioidomycosis. J Fungi. 2019;5(3):54. DOI: 10.3390/jof5030054
17. Pedroso VSP, Lyon AC, Araújo SA, Veloso JMR, Pedroso ERP, Teixeira AL. Paracoccidioidomycosis case series with and without central nervous system involvement. Rev Soc Bras Med Trop. 2012 Oct;45(5):586-90. DOI: 10.1590/S0037-86822012000500009
18. Almeida SM, Queiroz-Telles F, Teive HAG, Ribeiro CE, Werneck LC. Central nervous system paracoccidioidomycosis: clinical features and laboratorial findings. J Infect. 2004 Feb;48(2):193-8. DOI: 10.1016/j.jinf.2003.08.012
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















