Atypical presentation of cutaneous leishmaniasis in a pregnant woman
DOI:
https://doi.org/10.5935/2764-734X.e20240541Keywords:
Cutaneous leishmaniasis, Amphotericin B, Meglumine antimoniate, Pregnancy complications, Infections, Case reportAbstract
Cutaneous leishmaniasis most commonly presents as a single cutaneous ulcer, but there are less common clinical presentations. Here we report a rare manifestation of cutaneous leishmaniasis diagnosed in a 37-year-old pregnant woman who presented with an infiltrative, erythematous, raspberry-like lesion on the nasal dorsum of three months’ duration. No mucosal involvement was noted, but the patient complained of local pain and paresthesias. There was no history of immunosuppression or other comorbidities. Initially treated with amphotericin B, the patient experienced a relapse after eight months, at which time we decided to restart treatment with pentavalent antimonial. The delay in diagnosis caused by the atypical presentation of the disease led to a delay in treatment. We emphasize the importance of including cutaneous leishmaniasis in the differential diagnosis of any skin lesion in endemic areas.
Downloads
INTRODUCTION
Tegumentary leishmaniasis (TL) is an infectious, non-contagious disease caused by protozoa of the genus Leishmania transmitted by different species of hematophagous insects, such as sandflies. In Brazil, eight species of Leishmania that cause disease in humans have been described, covering a wide spectrum of manifestations that vary according to the characteristics of the host and the causative agent1,2. TL is classified into four main clinical forms: localized cutaneous leishmaniasis (which can be caused by any species of Leishmania), typically characterized by one or more ulcerated lesions with well-defined borders and a clean background; disseminated cutaneous leishmaniasis (caused mainly by the species Leishmania (Viannia) braziliensis, present throughout the national territory) with 20 or more acneiform lesions distributed in at least two distinct regions of the body surface; diffuse cutaneous leishmaniasis with multiple nodular lesions that do not ulcerate (caused solely by Leishmania (Leishmania) amazonensis, which is more prevalent in the northern region of Brazil); and mucosal leishmaniasis, characterized by involvement mainly of the nasal and/or oral mucosa (usually also caused by Leishmania (Viannia) braziliensis). Other species, such as Leishmania (Viannia) guyanensis, Leishmania (Viannia) shawi, Leishmania (Viannia) naiffi, Leishmania (Viannia) lainsoni, and Leishmania (Viannia) lindenberg circulate only in the Amazon region and mainly cause the localized cutaneous form3.
The localized cutaneous form of TL is the most common, with an ulcerated lesion being its main clinical manifestation. The herpetiform and raspberry-like forms of TL are rarely reported1 and, because they are atypical, they hamper clinical reasoning, resulting in delays in diagnosis and appropriate therapeutic management. The importance of this case report is that it alerts health professionals from different backgrounds about the occurrence of cutaneous leishmaniasis through unusual clinical manifestations.
CASE REPORT
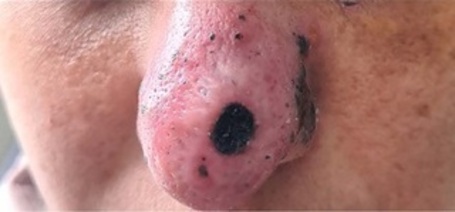
A 37-year-old female patient, born in Franco da Rocha (a municipality in the metropolitan region of São Paulo), presented with an infiltrative and hyperemic lesion on the nasal dorsum that appeared about 3 months ago. She worked as a commercial manager for a company but lived on a farm in the countryside. She was in the 24th week of pregnancy, with no reported comorbidities, under regular prenatal care and, until then, without any other complications. The skin condition began as a small puncture wound with an acneiform appearance on the nasal dorsum, which soon evolved into a shallow ulcer with irregular borders, increasing in size over 2 weeks. During the initial period, she used topical antibiotics and healing ointments that reduced the size of the ulcer. However, it was replaced by an erythematous infiltration that progressively extended from the dorsum to the nasal tip, associated with local pain and paresthesia. The region where the initial ulcer was located was replaced by a necrotic crust and her nose quickly acquired a raspberrylike shape (Figure 1). There were no systemic symptoms such as fever or weight loss. Lymphadenopathy or visceromegaly was not identified during the physical examination. The patient underwent an ear, nose, and throat evaluation, which found no evidence of nasal or oral mucosal involvement. A biopsy was performed, using a 4-mm punch, and the histopathological analysis of the fragment showed the presence of a diffuse granulomatous reaction with vasculopathy and plasmacytosis. Intramacrophage amastigote forms were visualized with hematoxylin and eosin staining (Figure 2), and an immunohistochemical study of the sample detected specific antigens (Figure 3). No DNA-test was carried out for Leishmania spp in the sample. On the other hand, direct detection of acid-fast bacilli and fungi was negative. Cultures for nonspecific aerobic bacteria, mycobacteria, and fungi were also negative. The search for autoantibodies ruled out autoimmune disease.
Figure 1. Infiltrative and hyperemic lesion on the dorsum and tip of the nose with a “raspberry-like” appearance.
Figure 2. Histopathological images of nasal dorsum skin biopsy material showing in (A): xanthomatous histiocyte-rich mononuclear infiltrate in the subepithelial dermis, stained in HE, 200× magnification; in (B): intracellular amastigote forms (arrows), in HE, 400× magnification; and in (C): intracellular amastigote forms (arrows), in HE, 1000× magnification (immersion). HE: Hematoxylin and Eosin.
Figure 3. Immunohistochemical microscopy images of nasal dorsum skin biopsy were positive for Leishmania spp antigens (circles), at (A) 200× magnification and (B) 400× magnification.
The patient was treated with intravenous liposomal amphotericin B at a dose of 3 mg/kg/day. On the seventh day of treatment, it had to be suspended due to hypokalemia. Considering that there had already been complete regression of the lesion with the total dose of 21 mg/kg of amphotericin, it was decided to permanently discontinue the drug and maintain outpatient follow-up. Eight months later, however, the patient returned with a recurrence of the infiltrative and hyperemic lesion on the nasal dorsum with an appearance similar to the previous one, although without ulcerations. It was then decided to re-treat her with intravenous pentavalent antimony at a dose of 15 mg/kg/day for 20 days, after which the lesion completely regressed, leaving only an atrophic scar (Figure 4). After another year of follow-up, there are no signs of recurrence of leishmaniasis, and the patient is about to be discharged from follow-up.
Figure 4. Resolution of the initial lesion, leaving only a small scar on the tip of the nose.
DISCUSSION
This case report challenges us to diagnose TL in one of its atypical and unusual forms: when it comes from non-endemic areas or has unusual clinical manifestations that share other etiologies in its differential diagnosis. Subcutaneous and deep mycoses, lymphoma and pseudolymphoma, and basal cell and squamous cell carcinoma, for example, are diseases that can mimic cutaneous leishmaniasis in its volcanic, luponoid, eczematous, erysipeloid, verrucous, dry, zosteriform, paronychic, sporotricoid, cancriform and annular forms. This occurs regardless of which part of the body is affected: the face, cheeks, ears, nose, eyelids, limbs, trunk, buttocks, palmoplantar or genital regions, and it sometimes concomitantly affects more than one area of the body4.
Although the reasons for the leishmaniasis polymorphism are not clear, the virulence of the parasite strain, the differences in the host’s defense mechanisms, and the patient’s degree of immunosuppression are important factors that influence the emergence of different presentations5. The immune response to leishmaniasis is complex and largely mediated by T cells, and generally requires the production of interferon-γ by Th1 cells to activate infected macrophages6. Diseases such as diabetes, neoplasms, and human immunodeficiency virus infection are risk factors for the development of a more severe form of TL. The ability of macrophages to effectively fight the intracellular parasite determines the extent of the disease.
The raspberry-like form of TL on the nasal dorsum of the patient in this case report was not associated with any known comorbidity or immunosuppression. However, she was pregnant at the end of the second trimester of pregnancy. It is known that pregnant women can all be considered a special population group due to the peculiar “immunological” condition related to pregnancy: by formatting a network of recognition, communication and repair, the maternal immune system prioritizes the maintenance of the well-being of the fetus. The fetus’s existence, in turn, modifies the way the mother responds to the environment, directly and indirectly affecting her immune response during pregnancy7. The fact is that pregnancy does not imply greater susceptibility to infectious diseases but modulates the immune system in such a way as to provoke differentiated responses to aggressions of the most diverse types, depending on the stage of pregnancy8,9. The repercussions of TL on human fetal health are not yet well established, while the adverse effects of vertical transmission are better documented in animal models10.
To confirm the etiology of a TL condition, making the appropriate diagnostic hypothesis to direct the request for relevant complementary tests is essential. Direct research in search of visualization of amastigote forms in lesional scrapings, tissue fragments, or impression smears (“imprints”) stained by the Giemsa method is still the gold standard method (with the help of optical microscopy) for the diagnosis of TL. On the other hand, a typical histopathological analysis of biopsied tissues shows diffuse granulomatous dermatitis ulcerated with lymphoplasmacytic infiltrate11 . When available, molecular biology plays an important role in diagnosis, as it has greater sensitivity and specificity than other tests, even when there is a low parasite load. It also allows the identification of the species responsible for the infection, guiding a more appropriate therapy with less risk of failure and recurrences12,13.
As for treatment, the drug of choice is pentavalent antimony, and the formulation with meglumine antimoniate is the only one available in Brazil. This drug, however, has important contraindications, including pregnancy, as it crosses the transplacental barrier. Additionally, it is a drug that should be avoided in patients over 50 years of age or those with heart disease or nephropathy. The second therapeutic option (used especially when there is some contraindication for antimony) is amphotericin B, preferably in the liposomal formulation, due to its lower toxicity2,13. This was the choice in the first treatment prescribed to our patient during pregnancy. However, with the recurrence of the disease after the pregnancy had already ended, meglumine antimoniate could be used for the new treatment. The dosage used in each of the treatments was adequate: according to the Brazilian Ministry of Health Manual, liposomal amphotericin B should be used at a dose of 2–5 mg/kg/day, with no limit on the maximum daily dose, up to a total dose of 25–40 mg/ kg, depending on the clinical response. Meglumine antimoniate, on the other hand, should be prescribed at a dose of 10–20 mg/kg/day for 20 days. We must also pay attention to its toxicity thresholds to avoid cardiac, hepatic, pancreatic, or renal changes that could necessitate modification or interruption of treatment1.
CONCLUSION
This report shows the importance of considering cutaneous leishmaniasis as a possible differential diagnosis in endemic areas, even when the lesions are not typical. The case also illustrates that pregnancy can play an important role in modulating immunogenicity, causing different responses by the body to certain infections. In terms of diagnosis, biopsy of the lesion for specific analyses remains the best method of identifying leishmanias. As for treatment, the available drugs still have limitations and side effects that need to be considered when making the choice.
References
1. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de Vigilância da Leishmaniose Tegumentar Americana. 1a ed. Brasília: Ministério da Saúde; 2017 [acesso em 2024 Fev 26]. Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar.pdf
2. Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8(4):419-33. DOI: 10.1586/eri.10.19
3. Marchi MNA de, Caldart ET, Martins FDC, Freire RL. Spatial analysis of leishmaniasis in Brazil: a systematized review. Rev Inst Med trop S Paulo. 2019;61:e68. DOI: 10.1590/S1678-9946201961068
4. Meireles CB, Maia LC, Soares GC, Teodoro IPP, Gadelha MDSV, da Silva CGL, et al. Atypical presentations of cutaneous leishmaniasis: a systematic review. Acta Trop. 2017:172:240-54. DOI: 10.1016/j.actatropica.2017.05.022
5. van Griensven J, Carrillo E, López-Vélez R, Lynen L, Moreno J. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect. 2014;20(4):286-99. DOI: 10.1111/1469-0691.12556
6. Von Stebut E. Immunology of cutaneous leishmaniasis: the role of mast cells, phagocytes and dendritic cells for protective immunity. Eur J Dermatol. 2007;17(2):115-22. DOI: 10.1684/ejd.2007.0122
7. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266-71. DOI: 10.1038/ni1037
8. Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425-33. DOI: 10.1111/j.1600-0897.2010.00836.x
9. Pokutnaya D, Shirzadi MR, Salari E, Molaei G. Cutaneous Leishmaniasis during Pregnancy, Preterm Birth, and Neonatal Death: A Case Report. Iran J Parasitol. 2020;15(4):608-14. DOI: 10.18502/ijpa.v15i4.4875
10. Morgan DJ, Guimaraes LH, Machado PR, D’Oliveira A Jr, Almeida RP, Lago EL, et al. Cutaneous leishmaniasis during pregnancy: exuberant lesions and potential fetal complications. Clin Infect Dis. 2007;45(4):478-82. DOI: 10.1086/520017
11. de Vries HJC, Schallig HD. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am J Clin Dermatol. 2022;23(6):823-40. DOI: 10.1007/s40257-022-00726-8
12. Graça GC, Volpini AC, Romero GA, Oliveira Neto MP, Hueb M, Porrozzi R, et al. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem Inst Oswaldo Cruz. 2012;107(5):664-74. DOI: 10.1590/s0074-02762012000500014
13. Almeida OL, Santos JB. Advances in the treatment of cutaneous leishmaniasis in the new world in the last ten years: a systematic literature review. An Bras Dermatol. 2011;86(3):497-506. DOI: 10.1590/s0365-05962011000300012
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Infections in Evidence

This work is licensed under a Creative Commons Attribution 4.0 International License.
The Copyright Transfer Agreement also understands that the authors guarantee that the respective Case Report has never been published in another communication vehicle or scientific journal. Papers presented at meetings and/or scientific congresses may be published in the electronic Journal INFECTIONS IN EVIDENCE - Case Reports, provided they have not been published in whole or in part in their Proceedings or Annals in the format of a complete article including images, discussion and bibliographic references, or that they have not been assigned a specific DOI number.
















